THE WATER BOOK


Water is an inorganic compound with the chemical formula H2O. It is a transparent, tasteless, odorless,[c] and nearly colorless chemical substance, and it is the main constituent of Earth‘s hydrosphere and the fluids of all known living organisms (in which it acts as a solvent[19]). It is vital for all known forms of life, despite not providing food energy or organic micronutrients. Its chemical formula, H2O, indicates that each of its molecules contains one oxygen and two hydrogen atoms, connected by covalent bonds. The hydrogen atoms are attached to the oxygen atom at an angle of 104.45°.[20] “Water” is also the name of the liquid state of H2O at standard temperature and pressure.
Because Earth’s environment is relatively close to water’s triple point, water exists on Earth as a solid, a liquid, and a gas.[21] It forms precipitation in the form of rain and aerosols in the form of fog. Clouds consist of suspended droplets of water and ice, its solid state. When finely divided, crystalline ice may precipitate in the form of snow. The gaseous state of water is steam or water vapor.
Water covers about 71% of the Earth’s surface, with seas and oceans making up most of the water volume (about 96.5%).[22] Small portions of water occur as groundwater (1.7%), in the glaciers and the ice caps of Antarctica and Greenland (1.7%), and in the air as vapor, clouds (consisting of ice and liquid water suspended in air), and precipitation (0.001%).[23][24] Water moves continually through the water cycle of evaporation, transpiration (evapotranspiration), condensation, precipitation, and runoff, usually reaching the sea.
Water plays an important role in the world economy. Approximately 70% of the freshwater used by humans goes to agriculture.[25] Fishing in salt and fresh water bodies has been, and continues to be, a major source of food for many parts of the world, providing 6.5% of global protein.[26] Much of the long-distance trade of commodities (such as oil, natural gas, and manufactured products) is transported by boats through seas, rivers, lakes, and canals. Large quantities of water, ice, and steam are used for cooling and heating in industry and homes. Water is an excellent solvent for a wide variety of substances, both mineral and organic; as such, it is widely used in industrial processes and in cooking and washing. Water, ice, and snow are also central to many sports and other forms of entertainment, such as swimming, pleasure boating, boat racing, surfing, sport fishing, diving, ice skating, snowboarding, and skiing.
Etymology
The word water comes from Old English wæter, from Proto-Germanic *watar (source also of Old Saxon watar, Old Frisian wetir, Dutch water, Old High German wazzar, German Wasser, vatn, Gothic 𐍅𐌰𐍄𐍉 (wato)), from Proto-Indo-European *wod-or, suffixed form of root *wed- (‘water‘; ‘wet‘).[27] Also cognate, through the Indo-European root, with Greek ύδωρ (ýdor; from Ancient Greek ὕδωρ (hýdōr), whence English ‘hydro-‘), Russian вода́ (vodá), Irish uisce, and Albanian ujë.
History
On Earth
One factor in estimating when water appeared on Earth is that water is continually being lost to space. H2O molecules in the atmosphere are broken up by photolysis, and the resulting free hydrogen atoms can sometimes escape Earth’s gravitational pull. When the Earth was younger and less massive, water would have been lost to space more easily. Lighter elements like hydrogen and helium are expected to leak from the atmosphere continually, but isotopic ratios of heavier noble gases in the modern atmosphere suggest that even the heavier elements in the early atmosphere were subject to significant losses.[28] In particular, xenon is useful for calculations of water loss over time. Not only is it a noble gas (and therefore is not removed from the atmosphere through chemical reactions with other elements), but comparisons between the abundances of its nine stable isotopes in the modern atmosphere reveal that the Earth lost at least one ocean of water early in its history, between the Hadean and Archean eons.[29][clarification needed]
Any water on Earth during the latter part of its accretion would have been disrupted by the Moon-forming impact (~4.5 billion years ago), which likely vaporized much of Earth’s crust and upper mantle and created a rock-vapor atmosphere around the young planet.[30][31] The rock vapor would have condensed within two thousand years, leaving behind hot volatiles which probably resulted in a majority carbon dioxide atmosphere with hydrogen and water vapor. Afterward, liquid water oceans may have existed despite the surface temperature of 230 °C (446 °F) due to the increased atmospheric pressure of the CO2 atmosphere. As the cooling continued, most CO2 was removed from the atmosphere by subduction and dissolution in ocean water, but levels oscillated wildly as new surface and mantle cycles appeared.[32]

Geological evidence also helps constrain the time frame for liquid water existing on Earth. A sample of pillow basalt (a type of rock formed during an underwater eruption) was recovered from the Isua Greenstone Belt and provides evidence that water existed on Earth 3.8 billion years ago.[33] In the Nuvvuagittuq Greenstone Belt, Quebec, Canada, rocks dated at 3.8 billion years old by one study[34] and 4.28 billion years old by another[35] show evidence of the presence of water at these ages.[33] If oceans existed earlier than this, any geological evidence has yet to be discovered (which may be because such potential evidence has been destroyed by geological processes like crustal recycling). More recently, in August 2020, researchers reported that sufficient water to fill the oceans may have always been on the Earth since the beginning of the planet’s formation.[36][37][38]
Unlike rocks, minerals called zircons are highly resistant to weathering and geological processes and so are used to understand conditions on the very early Earth. Mineralogical evidence from zircons has shown that liquid water and an atmosphere must have existed 4.404 ± 0.008 billion years ago, very soon after the formation of Earth.[39][40][41][42] This presents somewhat of a paradox, as the cool early Earth hypothesis suggests temperatures were cold enough to freeze water between about 4.4 billion and 4.0 billion years ago. Other studies of zircons found in Australian Hadean rock point to the existence of plate tectonics as early as 4 billion years ago. If true, that implies that rather than a hot, molten surface and an atmosphere full of carbon dioxide, early Earth’s surface was much as it is today (in terms of thermal insulation). The action of plate tectonics traps vast amounts of CO2, thereby reducing greenhouse effects, leading to a much cooler surface temperature and the formation of solid rock and liquid water.[43]Properties
Water (H2O) is a polar inorganic compound. At room temperature it is a tasteless and odorless liquid, nearly colorless with a hint of blue. This simplest hydrogen chalcogenide is by far the most studied chemical compound and is described as the “universal solvent” for its ability to dissolve many substances.[44][45] This allows it to be the “solvent of life”:[46] indeed, water as found in nature almost always includes various dissolved substances, and special steps are required to obtain chemically pure water. Water is the only common substance to exist as a solid, liquid, and gas in normal terrestrial conditions.[47]
States

Along with oxidane, water is one of the two official names for the chemical compound H
2O;[48] it is also the liquid phase of H
2O.[49] The other two common states of matter of water are the solid phase, ice, and the gaseous phase, water vapor or steam. The addition or removal of heat can cause phase transitions: freezing (water to ice), melting (ice to water), vaporization (water to vapor), condensation (vapor to water), sublimation (ice to vapor) and deposition (vapor to ice).[50]
Density
Water differs from most liquids in that it becomes less dense as it freezes.[d] In 1 atm pressure, it reaches its maximum density of 999.972 kg/m3 (62.4262 lb/cu ft) at 3.98 °C (39.16 °F), or almost 1,000 kg/m3 (62.43 lb/cu ft) at almost 4 °C (39 °F).[52][53] The density of ice is 917 kg/m3 (57.25 lb/cu ft), an expansion of 9%.[54][55] This expansion can exert enormous pressure, bursting pipes and cracking rocks.[56]
In a lake or ocean, water at 4 °C (39 °F) sinks to the bottom, and ice forms on the surface, floating on the liquid water. This ice insulates the water below, preventing it from freezing solid. Without this protection, most aquatic organisms residing in lakes would perish during the winter.[57]
Magnetism
Water is a diamagnetic material.[58] Though interaction is weak, with superconducting magnets it can attain a notable interaction.[58]
Phase transitions
At a pressure of one atmosphere (atm), ice melts or water freezes (solidifies) at 0 °C (32 °F) and water boils or vapor condenses at 100 °C (212 °F). However, even below the boiling point, water can change to vapor at its surface by evaporation (vaporization throughout the liquid is known as boiling). Sublimation and deposition also occur on surfaces.[50] For example, frost is deposited on cold surfaces while snowflakes form by deposition on an aerosol particle or ice nucleus.[59] In the process of freeze-drying, a food is frozen and then stored at low pressure so the ice on its surface sublimates.[60]
The melting and boiling points depend on pressure. A good approximation for the rate of change of the melting temperature with pressure is given by the Clausius–Clapeyron relation:

where �L


The Clausius-Clapeyron relation also applies to the boiling point, but with the liquid/gas transition the vapor phase has a much lower density than the liquid phase, so the boiling point increases with pressure.[63] Water can remain in a liquid state at high temperatures in the deep ocean or underground. For example, temperatures exceed 205 °C (401 °F) in Old Faithful, a geyser in Yellowstone National Park.[64] In hydrothermal vents, the temperature can exceed 400 °C (752 °F).[65]
At sea level, the boiling point of water is 100 °C (212 °F). As atmospheric pressure decreases with altitude, the boiling point decreases by 1 °C every 274 meters. High-altitude cooking takes longer than sea-level cooking. For example, at 1,524 metres (5,000 ft), cooking time must be increased by a fourth to achieve the desired result.[66] Conversely, a pressure cooker can be used to decrease cooking times by raising the boiling temperature.[67] In a vacuum, water will boil at room temperature.[68]
Triple and critical points

On a pressure/temperature phase diagram (see figure), there are curves separating solid from vapor, vapor from liquid, and liquid from solid. These meet at a single point called the triple point, where all three phases can coexist. The triple point is at a temperature of 273.16 K (0.01 °C; 32.02 °F) and a pressure of 611.657 pascals (0.00604 atm; 0.0887 psi);[69] it is the lowest pressure at which liquid water can exist. Until 2019, the triple point was used to define the Kelvin temperature scale.[70][71]
The water/vapor phase curve terminates at 647.096 K (373.946 °C; 705.103 °F) and 22.064 megapascals (3,200.1 psi; 217.75 atm).[72] This is known as the critical point. At higher temperatures and pressures the liquid and vapor phases form a continuous phase called a supercritical fluid. It can be gradually compressed or expanded between gas-like and liquid-like densities; its properties (which are quite different from those of ambient water) are sensitive to density. For example, for suitable pressures and temperatures it can mix freely with nonpolar compounds, including most organic compounds. This makes it useful in a variety of applications including high-temperature electrochemistry and as an ecologically benign solvent or catalyst in chemical reactions involving organic compounds. In Earth’s mantle, it acts as a solvent during mineral formation, dissolution and deposition.[73][74]
Phases of ice and water
The normal form of ice on the surface of Earth is ice Ih, a phase that forms crystals with hexagonal symmetry. Another with cubic crystalline symmetry, ice Ic, can occur in the upper atmosphere.[75] As the pressure increases, ice forms other crystal structures. As of 2019, seventeen have been experimentally confirmed and several more are predicted theoretically.[76] The eighteenth form of ice, ice XVIII, a face-centred-cubic, superionic ice phase, was discovered when a droplet of water was subject to a shock wave that raised the water’s pressure to millions of atmospheres and its temperature to thousands of degrees, resulting in a structure of rigid oxygen atoms in which hydrogen atoms flowed freely.[77][78] When sandwiched between layers of graphene, ice forms a square lattice.[79]
The details of the chemical nature of liquid water are not well understood; some theories suggest that its unusual behaviour is due to the existence of two liquid states.[53][80][81][82]
Taste and odor
Pure water is usually described as tasteless and odorless, although humans have specific sensors that can feel the presence of water in their mouths,[83][84] and frogs are known to be able to smell it.[85] However, water from ordinary sources (including mineral water) usually has many dissolved substances, that may give it varying tastes and odors. Humans and other animals have developed senses that enable them to evaluate the potability of water in order to avoid water that is too salty or putrid.[86]
Color and appearance
Pure water is visibly blue due to absorption of light in the region c. 600–800 nm.[87] The color can be easily observed in a glass of tap-water placed against a pure white background, in daylight. The principal absorption bands responsible for the color are overtones of the O–H stretching vibrations. The apparent intensity of the color increases with the depth of the water column, following Beer’s law. This also applies, for example, with a swimming pool when the light source is sunlight reflected from the pool’s white tiles.
In nature, the color may also be modified from blue to green due to the presence of suspended solids or algae.
In industry, near-infrared spectroscopy is used with aqueous solutions as the greater intensity of the lower overtones of water means that glass cuvettes with short path-length may be employed. To observe the fundamental stretching absorption spectrum of water or of an aqueous solution in the region around 3,500 cm−1 (2.85 μm)[88] a path length of about 25 μm is needed. Also, the cuvette must be both transparent around 3500 cm−1 and insoluble in water; calcium fluoride is one material that is in common use for the cuvette windows with aqueous solutions.
The Raman-active fundamental vibrations may be observed with, for example, a 1 cm sample cell.
Aquatic plants, algae, and other photosynthetic organisms can live in water up to hundreds of meters deep, because sunlight can reach them. Practically no sunlight reaches the parts of the oceans below 1,000 meters (3,300 ft) of depth.
The refractive index of liquid water (1.333 at 20 °C (68 °F)) is much higher than that of air (1.0), similar to those of alkanes and ethanol, but lower than those of glycerol (1.473), benzene (1.501), carbon disulfide (1.627), and common types of glass (1.4 to 1.6). The refraction index of ice (1.31) is lower than that of liquid water.
Molecular polarity

In a water molecule, the hydrogen atoms form a 104.5° angle with the oxygen atom. The hydrogen atoms are close to two corners of a tetrahedron centered on the oxygen. At the other two corners are lone pairs of valence electrons that do not participate in the bonding. In a perfect tetrahedron, the atoms would form a 109.5° angle, but the repulsion between the lone pairs is greater than the repulsion between the hydrogen atoms.[89][90] The O–H bond length is about 0.096 nm.[91]
Other substances have a tetrahedral molecular structure, for example, methane (CH
4) and hydrogen sulfide (H
2S). However, oxygen is more electronegative than most other elements, so the oxygen atom retains a negative charge while the hydrogen atoms are positively charged. Along with the bent structure, this gives the molecule an electrical dipole moment and it is classified as a polar molecule.[92]
Water is a good polar solvent, dissolving many salts and hydrophilic organic molecules such as sugars and simple alcohols such as ethanol. Water also dissolves many gases, such as oxygen and carbon dioxide—the latter giving the fizz of carbonated beverages, sparkling wines and beers. In addition, many substances in living organisms, such as proteins, DNA and polysaccharides, are dissolved in water. The interactions between water and the subunits of these biomacromolecules shape protein folding, DNA base pairing, and other phenomena crucial to life (hydrophobic effect).
Many organic substances (such as fats and oils and alkanes) are hydrophobic, that is, insoluble in water. Many inorganic substances are insoluble too, including most metal oxides, sulfides, and silicates.
Hydrogen bonding
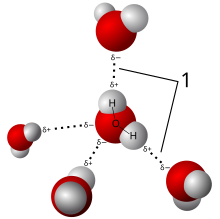
Because of its polarity, a molecule of water in the liquid or solid state can form up to four hydrogen bonds with neighboring molecules. Hydrogen bonds are about ten times as strong as the Van der Waals force that attracts molecules to each other in most liquids. This is the reason why the melting and boiling points of water are much higher than those of other analogous compounds like hydrogen sulfide. They also explain its exceptionally high specific heat capacity (about 4.2 J/(g·K)), heat of fusion (about 333 J/g), heat of vaporization (2257 J/g), and thermal conductivity (between 0.561 and 0.679 W/(m·K)). These properties make water more effective at moderating Earth’s climate, by storing heat and transporting it between the oceans and the atmosphere. The hydrogen bonds of water are around 23 kJ/mol (compared to a covalent O-H bond at 492 kJ/mol). Of this, it is estimated that 90% is attributable to electrostatics, while the remaining 10% is partially covalent.[93]
These bonds are the cause of water’s high surface tension[94] and capillary forces. The capillary action refers to the tendency of water to move up a narrow tube against the force of gravity. This property is relied upon by all vascular plants, such as trees.[citation needed]

Self-ionization
Water is a weak solution of hydronium hydroxide—there is an equilibrium 2H
2O ⇌ H
3O+
+ OH−
, in combination with solvation of the resulting hydronium and hydroxide ions.
Electrical conductivity and electrolysis
Pure water has a low electrical conductivity, which increases with the dissolution of a small amount of ionic material such as common salt.
Liquid water can be split into the elements hydrogen and oxygen by passing an electric current through it—a process called electrolysis. The decomposition requires more energy input than the heat released by the inverse process (285.8 kJ/mol, or 15.9 MJ/kg).[96]
Mechanical properties
Liquid water can be assumed to be incompressible for most purposes: its compressibility ranges from 4.4 to 5.1×10−10 Pa−1 in ordinary conditions.[97] Even in oceans at 4 km depth, where the pressure is 400 atm, water suffers only a 1.8% decrease in volume.[98]
The viscosity of water is about 10−3 Pa·s or 0.01 poise at 20 °C (68 °F), and the speed of sound in liquid water ranges between 1,400 and 1,540 meters per second (4,600 and 5,100 ft/s) depending on temperature. Sound travels long distances in water with little attenuation, especially at low frequencies (roughly 0.03 dB/km for 1 kHz), a property that is exploited by cetaceans and humans for communication and environment sensing (sonar).[99]
Reactivity
Metallic elements which are more electropositive than hydrogen, particularly the alkali metals and alkaline earth metals such as lithium, sodium, calcium, potassium and cesium displace hydrogen from water, forming hydroxides and releasing hydrogen. At high temperatures, carbon reacts with steam to form carbon monoxide and hydrogen.[citation needed]
On Earth
Hydrology is the study of the movement, distribution, and quality of water throughout the Earth. The study of the distribution of water is hydrography. The study of the distribution and movement of groundwater is hydrogeology, of glaciers is glaciology, of inland waters is limnology and distribution of oceans is oceanography. Ecological processes with hydrology are in the focus of ecohydrology.
The collective mass of water found on, under, and over the surface of a planet is called the hydrosphere. Earth’s approximate water volume (the total water supply of the world) is 1.386 billion cubic kilometres (333 million cubic miles).[23]
Liquid water is found in bodies of water, such as an ocean, sea, lake, river, stream, canal, pond, or puddle. The majority of water on Earth is seawater. Water is also present in the atmosphere in solid, liquid, and vapor states. It also exists as groundwater in aquifers.
Water is important in many geological processes. Groundwater is present in most rocks, and the pressure of this groundwater affects patterns of faulting. Water in the mantle is responsible for the melt that produces volcanoes at subduction zones. On the surface of the Earth, water is important in both chemical and physical weathering processes. Water, and to a lesser but still significant extent, ice, are also responsible for a large amount of sediment transport that occurs on the surface of the earth. Deposition of transported sediment forms many types of sedimentary rocks, which make up the geologic record of Earth history.
Water cycle

The water cycle (known scientifically as the hydrologic cycle) is the continuous exchange of water within the hydrosphere, between the atmosphere, soil water, surface water, groundwater, and plants.
Water moves perpetually through each of these regions in the water cycle consisting of the following transfer processes:
- evaporation from oceans and other water bodies into the air and transpiration from land plants and animals into the air.
- precipitation, from water vapor condensing from the air and falling to the earth or ocean.
- runoff from the land usually reaching the sea.
Most water vapors found mostly in the ocean returns to it, but winds carry water vapor over land at the same rate as runoff into the sea, about 47 Tt per year whilst evaporation and transpiration happening in land masses also contribute another 72 Tt per year. Precipitation, at a rate of 119 Tt per year over land, has several forms: most commonly rain, snow, and hail, with some contribution from fog and dew.[100] Dew is small drops of water that are condensed when a high density of water vapor meets a cool surface. Dew usually forms in the morning when the temperature is the lowest, just before sunrise and when the temperature of the earth’s surface starts to increase.[101] Condensed water in the air may also refract sunlight to produce rainbows.
Water runoff often collects over watersheds flowing into rivers. Through erosion, runoff shapes the environment creating river valleys and deltas which provide rich soil and level ground for the establishment of population centers. A flood occurs when an area of land, usually low-lying, is covered with water which occurs when a river overflows its banks or a storm surge happens. On the other hand, drought is an extended period of months or years when a region notes a deficiency in its water supply. This occurs when a region receives consistently below average precipitation either due to its topography or due to its location in terms of latitude.
Water resources
Water resources are natural resources of water that are potentially useful for humans,[102] for example as a source of drinking water supply or irrigation water. Water occurs as both “stocks” and “flows”. Water can be stored as lakes, water vapor, groundwater or aquifers, and ice and snow. Of the total volume of global freshwater, an estimated 69 percent is stored in glaciers and permanent snow cover; 30 percent is in groundwater; and the remaining 1 percent in lakes, rivers, the atmosphere, and biota.[103] The length of time water remains in storage is highly variable: some aquifers consist of water stored over thousands of years but lake volumes may fluctuate on a seasonal basis, decreasing during dry periods and increasing during wet ones. A substantial fraction of the water supply for some regions consists of water extracted from water stored in stocks, and when withdrawals exceed recharge, stocks decrease. By some estimates, as much as 30 percent of total water used for irrigation comes from unsustainable withdrawals of groundwater, causing groundwater depletion.[104]
Seawater and tides
Seawater contains about 3.5% sodium chloride on average, plus smaller amounts of other substances. The physical properties of seawater differ from fresh water in some important respects. It freezes at a lower temperature (about −1.9 °C (28.6 °F)) and its density increases with decreasing temperature to the freezing point, instead of reaching maximum density at a temperature above freezing. The salinity of water in major seas varies from about 0.7% in the Baltic Sea to 4.0% in the Red Sea. (The Dead Sea, known for its ultra-high salinity levels of between 30 and 40%, is really a salt lake.)
Tides are the cyclic rising and falling of local sea levels caused by the tidal forces of the Moon and the Sun acting on the oceans. Tides cause changes in the depth of the marine and estuarine water bodies and produce oscillating currents known as tidal streams. The changing tide produced at a given location is the result of the changing positions of the Moon and Sun relative to the Earth coupled with the effects of Earth rotation and the local bathymetry. The strip of seashore that is submerged at high tide and exposed at low tide, the intertidal zone, is an important ecological product of ocean tides.
Effects on life

From a biological standpoint, water has many distinct properties that are critical for the proliferation of life. It carries out this role by allowing organic compounds to react in ways that ultimately allow replication. All known forms of life depend on water. Water is vital both as a solvent in which many of the body’s solutes dissolve and as an essential part of many metabolic processes within the body. Metabolism is the sum total of anabolism and catabolism. In anabolism, water is removed from molecules (through energy requiring enzymatic chemical reactions) in order to grow larger molecules (e.g., starches, triglycerides, and proteins for storage of fuels and information). In catabolism, water is used to break bonds in order to generate smaller molecules (e.g., glucose, fatty acids, and amino acids to be used for fuels for energy use or other purposes). Without water, these particular metabolic processes could not exist.
Water is fundamental to both photosynthesis and respiration. Photosynthetic cells use the sun’s energy to split off water’s hydrogen from oxygen.[105] In the presence of sunlight, hydrogen is combined with CO
2 (absorbed from air or water) to form glucose and release oxygen.[106] All living cells use such fuels and oxidize the hydrogen and carbon to capture the sun’s energy and reform water and CO
2 in the process (cellular respiration).
Water is also central to acid-base neutrality and enzyme function. An acid, a hydrogen ion (H+
, that is, a proton) donor, can be neutralized by a base, a proton acceptor such as a hydroxide ion (OH−
) to form water. Water is considered to be neutral, with a pH (the negative log of the hydrogen ion concentration) of 7. Acids have pH values less than 7 while bases have values greater than 7.
Aquatic life forms
Earth’s surface waters are filled with life. The earliest life forms appeared in water; nearly all fish live exclusively in water, and there are many types of marine mammals, such as dolphins and whales. Some kinds of animals, such as amphibians, spend portions of their lives in water and portions on land. Plants such as kelp and algae grow in the water and are the basis for some underwater ecosystems. Plankton is generally the foundation of the ocean food chain.
Aquatic vertebrates must obtain oxygen to survive, and they do so in various ways. Fish have gills instead of lungs, although some species of fish, such as the lungfish, have both. Marine mammals, such as dolphins, whales, otters, and seals need to surface periodically to breathe air. Some amphibians are able to absorb oxygen through their skin. Invertebrates exhibit a wide range of modifications to survive in poorly oxygenated waters including breathing tubes (see insect and mollusc siphons) and gills (Carcinus). However, as invertebrate life evolved in an aquatic habitat most have little or no specialization for respiration in water.
- Some of the biodiversity of a coral reef
- Some marine diatoms – a key phytoplankton group
- Squat lobster and Alvinocarididae shrimp at the Von Damm hydrothermal field survive by altered water chemistry.
Effects on human civilization
This section needs additional citations for verification. Please help improve this article by adding citations to reliable sources in this section. Unsourced material may be challenged and removed. (May 2018) (Learn how and when to remove this template message) |

Civilization has historically flourished around rivers and major waterways; Mesopotamia, one of the so-called cradles of civilization, was situated between the major rivers Tigris and Euphrates; the ancient society of the Egyptians depended entirely upon the Nile. The early Indus Valley civilization (c. 3300 BCE – c. 1300 BCE) developed along the Indus River and tributaries that flowed out of the Himalayas. Rome was also founded on the banks of the Italian river Tiber. Large metropolises like Rotterdam, London, Montreal, Paris, New York City, Buenos Aires, Shanghai, Tokyo, Chicago, and Hong Kong owe their success in part to their easy accessibility via water and the resultant expansion of trade. Islands with safe water ports, like Singapore, have flourished for the same reason. In places such as North Africa and the Middle East, where water is more scarce, access to clean drinking water was and is a major factor in human development.
Health and pollution

Water fit for human consumption is called drinking water or potable water. Water that is not potable may be made potable by filtration or distillation, or by a range of other methods. More than 660 million people do not have access to safe drinking water.[107][108]
Water that is not fit for drinking but is not harmful to humans when used for swimming or bathing is called by various names other than potable or drinking water, and is sometimes called safe water, or “safe for bathing”. Chlorine is a skin and mucous membrane irritant that is used to make water safe for bathing or drinking. Its use is highly technical and is usually monitored by government regulations (typically 1 part per million (ppm) for drinking water, and 1–2 ppm of chlorine not yet reacted with impurities for bathing water). Water for bathing may be maintained in satisfactory microbiological condition using chemical disinfectants such as chlorine or ozone or by the use of ultraviolet light.
Water reclamation is the process of converting wastewater (most commonly sewage, also called municipal wastewater) into water that can be reused for other purposes. There are 2.3 billion people who reside in nations with water scarcities, which means that each individual receives less than 1,700 cubic metres (60,000 cu ft) of water annually. 380 billion cubic metres (13×1012 cu ft) of municipal wastewater are produced globally each year.[109][110][111]
Freshwater is a renewable resource, recirculated by the natural hydrologic cycle, but pressures over access to it result from the naturally uneven distribution in space and time, growing economic demands by agriculture and industry, and rising populations. Currently, nearly a billion people around the world lack access to safe, affordable water. In 2000, the United Nations established the Millennium Development Goals for water to halve by 2015 the proportion of people worldwide without access to safe water and sanitation. Progress toward that goal was uneven, and in 2015 the UN committed to the Sustainable Development Goals of achieving universal access to safe and affordable water and sanitation by 2030. Poor water quality and bad sanitation are deadly; some five million deaths a year are caused by water-related diseases. The World Health Organization estimates that safe water could prevent 1.4 million child deaths from diarrhoea each year.[112]
In developing countries, 90% of all municipal wastewater still goes untreated into local rivers and streams.[113] Some 50 countries, with roughly a third of the world’s population, also suffer from medium or high water scarcity and 17 of these extract more water annually than is recharged through their natural water cycles.[114] The strain not only affects surface freshwater bodies like rivers and lakes, but it also degrades groundwater resources.
Human uses

Agriculture
The most substantial human use of water is for agriculture, including irrigated agriculture, which accounts for as much as 80 to 90 percent of total human water consumption.[116] In the United States, 42% of freshwater withdrawn for use is for irrigation, but the vast majority of water “consumed” (used and not returned to the environment) goes to agriculture.[117]
Access to fresh water is often taken for granted, especially in developed countries that have built sophisticated water systems for collecting, purifying, and delivering water, and removing wastewater. But growing economic, demographic, and climatic pressures are increasing concerns about water issues, leading to increasing competition for fixed water resources, giving rise to the concept of peak water.[118] As populations and economies continue to grow, consumption of water-thirsty meat expands, and new demands rise for biofuels or new water-intensive industries, new water challenges are likely.[119]
An assessment of water management in agriculture was conducted in 2007 by the International Water Management Institute in Sri Lanka to see if the world had sufficient water to provide food for its growing population.[120] It assessed the current availability of water for agriculture on a global scale and mapped out locations suffering from water scarcity. It found that a fifth of the world’s people, more than 1.2 billion, live in areas of physical water scarcity, where there is not enough water to meet all demands. A further 1.6 billion people live in areas experiencing economic water scarcity, where the lack of investment in water or insufficient human capacity make it impossible for authorities to satisfy the demand for water. The report found that it would be possible to produce the food required in the future, but that continuation of today’s food production and environmental trends would lead to crises in many parts of the world. To avoid a global water crisis, farmers will have to strive to increase productivity to meet growing demands for food, while industries and cities find ways to use water more efficiently.[121]
Water scarcity is also caused by production of water intensive products. For example, cotton: 1 kg of cotton—equivalent of a pair of jeans—requires 10.9 cubic meters (380 cu ft) water to produce. While cotton accounts for 2.4% of world water use, the water is consumed in regions that are already at a risk of water shortage. Significant environmental damage has been caused: for example, the diversion of water by the former Soviet Union from the Amu Darya and Syr Darya rivers to produce cotton was largely responsible for the disappearance of the Aral Sea.[122]
- Water requirement per tonne of food product
- Water distribution in subsurface drip irrigation
- Irrigation of field crops
As a scientific standard
On 7 April 1795, the gram was defined in France to be equal to “the absolute weight of a volume of pure water equal to a cube of one-hundredth of a meter, and at the temperature of melting ice”.[123] For practical purposes though, a metallic reference standard was required, one thousand times more massive, the kilogram. Work was therefore commissioned to determine precisely the mass of one liter of water. In spite of the fact that the decreed definition of the gram specified water at 0 °C (32 °F)—a highly reproducible temperature—the scientists chose to redefine the standard and to perform their measurements at the temperature of highest water density, which was measured at the time as 4 °C (39 °F).[124]
The Kelvin temperature scale of the SI system was based on the triple point of water, defined as exactly 273.16 K (0.01 °C; 32.02 °F), but as of May 2019 is based on the Boltzmann constant instead. The scale is an absolute temperature scale with the same increment as the Celsius temperature scale, which was originally defined according to the boiling point (set to 100 °C (212 °F)) and melting point (set to 0 °C (32 °F)) of water.
Natural water consists mainly of the isotopes hydrogen-1 and oxygen-16, but there is also a small quantity of heavier isotopes oxygen-18, oxygen-17, and hydrogen-2 (deuterium). The percentage of the heavier isotopes is very small, but it still affects the properties of water. Water from rivers and lakes tends to contain less heavy isotopes than seawater. Therefore, standard water is defined in the Vienna Standard Mean Ocean Water specification.
For drinking



The human body contains from 55% to 78% water, depending on body size.[125][user-generated source?] To function properly, the body requires between one and seven liters (0.22 and 1.54 imp gal; 0.26 and 1.85 U.S. gal)[citation needed] of water per day to avoid dehydration; the precise amount depends on the level of activity, temperature, humidity, and other factors. Most of this is ingested through foods or beverages other than drinking straight water. It is not clear how much water intake is needed by healthy people, though the British Dietetic Association advises that 2.5 liters of total water daily is the minimum to maintain proper hydration, including 1.8 liters (6 to 7 glasses) obtained directly from beverages.[126] Medical literature favors a lower consumption, typically 1 liter of water for an average male, excluding extra requirements due to fluid loss from exercise or warm weather.[127]
Healthy kidneys can excrete 0.8 to 1 liter of water per hour, but stress such as exercise can reduce this amount. People can drink far more water than necessary while exercising, putting them at risk of water intoxication (hyperhydration), which can be fatal.[128][129] The popular claim that “a person should consume eight glasses of water per day” seems to have no real basis in science.[130] Studies have shown that extra water intake, especially up to 500 milliliters (18 imp fl oz; 17 U.S. fl oz) at mealtime, was associated with weight loss.[131][132][133][134][135][136] Adequate fluid intake is helpful in preventing constipation.[137]

An original recommendation for water intake in 1945 by the Food and Nutrition Board of the U.S. National Research Council read: “An ordinary standard for diverse persons is 1 milliliter for each calorie of food. Most of this quantity is contained in prepared foods.”[138] The latest dietary reference intake report by the U.S. National Research Council in general recommended, based on the median total water intake from US survey data (including food sources): 3.7 liters (0.81 imp gal; 0.98 U.S. gal) for men and 2.7 liters (0.59 imp gal; 0.71 U.S. gal) of water total for women, noting that water contained in food provided approximately 19% of total water intake in the survey.[139]
Specifically, pregnant and breastfeeding women need additional fluids to stay hydrated. The US Institute of Medicine recommends that, on average, men consume 3 liters (0.66 imp gal; 0.79 U.S. gal) and women 2.2 liters (0.48 imp gal; 0.58 U.S. gal); pregnant women should increase intake to 2.4 liters (0.53 imp gal; 0.63 U.S. gal) and breastfeeding women should get 3 liters (12 cups), since an especially large amount of fluid is lost during nursing.[140] Also noted is that normally, about 20% of water intake comes from food, while the rest comes from drinking water and beverages (caffeinated included). Water is excreted from the body in multiple forms; through urine and feces, through sweating, and by exhalation of water vapor in the breath. With physical exertion and heat exposure, water loss will increase and daily fluid needs may increase as well.
Humans require water with few impurities. Common impurities include metal salts and oxides, including copper, iron, calcium and lead,[141][full citation needed] and harmful bacteria, such as Vibrio. Some solutes are acceptable and even desirable for taste enhancement and to provide needed electrolytes.[142]
The single largest (by volume) freshwater resource suitable for drinking is Lake Baikal in Siberia.[143]
Washing

Washing is a method of cleaning, usually with water and soap or detergent. Washing and then rinsing both body and clothing is an essential part of good hygiene and health. [citation needed]
Often people use soaps and detergents to assist in the emulsification of oils and dirt particles so they can be washed away. The soap can be applied directly, or with the aid of a washcloth.
People wash themselves, or bathe periodically for religious ritual or therapeutic purposes[144] or as a recreational activity.
In Europe, some people use a bidet to wash their external genitalia and the anal region after using the toilet, instead of using toilet paper.[145] The bidet is common in predominantly Catholic countries where water is considered essential for anal cleansing.[146]
More frequent is washing of just the hands, e.g. before and after preparing food and eating, after using the toilet, after handling something dirty, etc. Hand washing is important in reducing the spread of germs.[147][148] Also common is washing the face, which is done after waking up, or to keep oneself cool during the day. Brushing one’s teeth is also essential for hygiene and is a part of washing.
‘Washing’ can also refer to the washing of clothing or other cloth items, like bedsheets, whether by hand or with a washing machine. It can also refer to washing one’s car, by lathering the exterior with car soap, then rinsing it off with a hose, or washing cookware.

Transportation
Maritime transport (or ocean transport) or more generally waterborne transport, is the transport of people (passengers) or goods (cargo) via waterways. Freight transport by sea has been widely used throughout recorded history. The advent of aviation has diminished the importance of sea travel for passengers, though it is still popular for short trips and pleasure cruises. Transport by water is cheaper than transport by air or ground,[151] but significantly slower for longer distances. Maritime transport accounts for roughly 80% of international trade, according to UNCTAD in 2020.
Maritime transport can be realized over any distance by boat, ship, sailboat or barge, over oceans and lakes, through canals or along rivers. Shipping may be for commerce, recreation, or military purposes. While extensive inland shipping is less critical today, the major waterways of the world including many canals are still very important and are integral parts of worldwide economies. Particularly, especially any material can be moved by water; however, water transport becomes impractical when material delivery is time-critical such as various types of perishable produce. Still, water transport is highly cost effective with regular schedulable cargoes, such as trans-oceanic shipping of consumer products – and especially for heavy loads or bulk cargos, such as coal, coke, ores, or grains. Arguably, the industrial revolution had its first impacts where cheap water transport by canal, navigations, or shipping by all types of watercraft on natural waterways supported cost-effective bulk transport.
Containerization revolutionized maritime transport starting in the 1970s. “General cargo” includes goods packaged in boxes, cases, pallets, and barrels. When a cargo is carried in more than one mode, it is intermodal or co-modal.Chemical uses
Water is widely used in chemical reactions as a solvent or reactant and less commonly as a solute or catalyst. In inorganic reactions, water is a common solvent, dissolving many ionic compounds, as well as other polar compounds such as ammonia and compounds closely related to water. In organic reactions, it is not usually used as a reaction solvent, because it does not dissolve the reactants well and is amphoteric (acidic and basic) and nucleophilic. Nevertheless, these properties are sometimes desirable. Also, acceleration of Diels-Alder reactions by water has been observed. Supercritical water has recently been a topic of research. Oxygen-saturated supercritical water combusts organic pollutants efficiently.
Heat exchange
Water and steam are a common fluid used for heat exchange, due to its availability and high heat capacity, both for cooling and heating. Cool water may even be naturally available from a lake or the sea. It is especially effective to transport heat through vaporization and condensation of water because of its large latent heat of vaporization. A disadvantage is that metals commonly found in industries such as steel and copper are oxidized faster by untreated water and steam. In almost all thermal power stations, water is used as the working fluid (used in a closed-loop between boiler, steam turbine, and condenser), and the coolant (used to exchange the waste heat to a water body or carry it away by evaporation in a cooling tower). In the United States, cooling power plants is the largest use of water.[152]
In the nuclear power industry, water can also be used as a neutron moderator. In most nuclear reactors, water is both a coolant and a moderator. This provides something of a passive safety measure, as removing the water from the reactor also slows the nuclear reaction down. However other methods are favored for stopping a reaction and it is preferred to keep the nuclear core covered with water so as to ensure adequate cooling.
Fire considerations

Water has a high heat of vaporization and is relatively inert, which makes it a good fire extinguishing fluid. The evaporation of water carries heat away from the fire. It is dangerous to use water on fires involving oils and organic solvents because many organic materials float on water and the water tends to spread the burning liquid.
Use of water in fire fighting should also take into account the hazards of a steam explosion, which may occur when water is used on very hot fires in confined spaces, and of a hydrogen explosion, when substances which react with water, such as certain metals or hot carbon such as coal, charcoal, or coke graphite, decompose the water, producing water gas.
The power of such explosions was seen in the Chernobyl disaster, although the water involved in this case did not come from fire-fighting but from the reactor’s own water cooling system. A steam explosion occurred when the extreme overheating of the core caused water to flash into steam. A hydrogen explosion may have occurred as a result of a reaction between steam and hot zirconium.
Some metallic oxides, most notably those of alkali metals and alkaline earth metals, produce so much heat in reaction with water that a fire hazard can develop. The alkaline earth oxide quicklime, also known as calcium oxide, is a mass-produced substance that is often transported in paper bags. If these are soaked through, they may ignite as their contents react with water.[153]
Recreation

Humans use water for many recreational purposes, as well as for exercising and for sports. Some of these include swimming, waterskiing, boating, surfing and diving. In addition, some sports, like ice hockey and ice skating, are played on ice. Lakesides, beaches and water parks are popular places for people to go to relax and enjoy recreation. Many find the sound and appearance of flowing water to be calming, and fountains and other flowing water structures are popular decorations. Some keep fish and other flora and fauna inside aquariums or ponds for show, fun, and companionship. Humans also use water for snow sports such as skiing, sledding, snowmobiling or snowboarding, which require the water to be at a low temperature either as ice or crystallized into snow.
Water industry
The water industry provides drinking water and wastewater services (including sewage treatment) to households and industry. Water supply facilities include water wells, cisterns for rainwater harvesting, water supply networks, and water purification facilities, water tanks, water towers, water pipes including old aqueducts. Atmospheric water generators are in development.
Drinking water is often collected at springs, extracted from artificial borings (wells) in the ground, or pumped from lakes and rivers. Building more wells in adequate places is thus a possible way to produce more water, assuming the aquifers can supply an adequate flow. Other water sources include rainwater collection. Water may require purification for human consumption. This may involve the removal of undissolved substances, dissolved substances and harmful microbes. Popular methods are filtering with sand which only removes undissolved material, while chlorination and boiling kill harmful microbes. Distillation does all three functions. More advanced techniques exist, such as reverse osmosis. Desalination of abundant seawater is a more expensive solution used in coastal arid climates.
The distribution of drinking water is done through municipal water systems, tanker delivery or as bottled water. Governments in many countries have programs to distribute water to the needy at no charge.
Reducing usage by using drinking (potable) water only for human consumption is another option. In some cities such as Hong Kong, seawater is extensively used for flushing toilets citywide in order to conserve freshwater resources.
Polluting water may be the biggest single misuse of water; to the extent that a pollutant limits other uses of the water, it becomes a waste of the resource, regardless of benefits to the polluter. Like other types of pollution, this does not enter standard accounting of market costs, being conceived as externalities for which the market cannot account. Thus other people pay the price of water pollution, while the private firms’ profits are not redistributed to the local population, victims of this pollution. Pharmaceuticals consumed by humans often end up in the waterways and can have detrimental effects on aquatic life if they bioaccumulate and if they are not biodegradable.
Municipal and industrial wastewater are typically treated at wastewater treatment plants. Mitigation of polluted surface runoff is addressed through a variety of prevention and treatment techniques.
- A water-carrier in India, 1882. In many places where running water is not available, water has to be transported by people.
- A manual water pump in China
- Water purification facility
Industrial applications
Many industrial processes rely on reactions using chemicals dissolved in water, suspension of solids in water slurries or using water to dissolve and extract substances, or to wash products or process equipment. Processes such as mining, chemical pulping, pulp bleaching, paper manufacturing, textile production, dyeing, printing, and cooling of power plants use large amounts of water, requiring a dedicated water source, and often cause significant water pollution.
Water is used in power generation. Hydroelectricity is electricity obtained from hydropower. Hydroelectric power comes from water driving a water turbine connected to a generator. Hydroelectricity is a low-cost, non-polluting, renewable energy source. The energy is supplied by the motion of water. Typically a dam is constructed on a river, creating an artificial lake behind it. Water flowing out of the lake is forced through turbines that turn generators.
Pressurized water is used in water blasting and water jet cutters. High pressure water guns are used for precise cutting. It works very well, is relatively safe, and is not harmful to the environment. It is also used in the cooling of machinery to prevent overheating, or prevent saw blades from overheating.
Water is also used in many industrial processes and machines, such as the steam turbine and heat exchanger, in addition to its use as a chemical solvent. Discharge of untreated water from industrial uses is pollution. Pollution includes discharged solutes (chemical pollution) and discharged coolant water (thermal pollution). Industry requires pure water for many applications and uses a variety of purification techniques both in water supply and discharge.
Food processing


Boiling, steaming, and simmering are popular cooking methods that often require immersing food in water or its gaseous state, steam.[154] Water is also used for dishwashing. Water also plays many critical roles within the field of food science.
Solutes such as salts and sugars found in water affect the physical properties of water. The boiling and freezing points of water are affected by solutes, as well as air pressure, which is in turn affected by altitude. Water boils at lower temperatures with the lower air pressure that occurs at higher elevations. One mole of sucrose (sugar) per kilogram of water raises the boiling point of water by 0.51 °C (0.918 °F), and one mole of salt per kg raises the boiling point by 1.02 °C (1.836 °F); similarly, increasing the number of dissolved particles lowers water’s freezing point.[155]
Solutes in water also affect water activity that affects many chemical reactions and the growth of microbes in food.[156] Water activity can be described as a ratio of the vapor pressure of water in a solution to the vapor pressure of pure water.[155] Solutes in water lower water activity—this is important to know because most bacterial growth ceases at low levels of water activity.[156] Not only does microbial growth affect the safety of food, but also the preservation and shelf life of food.
Water hardness is also a critical factor in food processing and may be altered or treated by using a chemical ion exchange system. It can dramatically affect the quality of a product, as well as playing a role in sanitation. Water hardness is classified based on concentration of calcium carbonate the water contains. Water is classified as soft if it contains less than 100 mg/L (UK)[157] or less than 60 mg/L (US).[158]
According to a report published by the Water Footprint organization in 2010, a single kilogram of beef requires 15 thousand liters (3.3×103 imp gal; 4.0×103 U.S. gal) of water; however, the authors also make clear that this is a global average and circumstantial factors determine the amount of water used in beef production.[159]
Medical use
Water for injection is on the World Health Organization‘s list of essential medicines.[160]
Distribution in nature
In the universe

Much of the universe’s water is produced as a byproduct of star formation. The formation of stars is accompanied by a strong outward wind of gas and dust. When this outflow of material eventually impacts the surrounding gas, the shock waves that are created compress and heat the gas. The water observed is quickly produced in this warm dense gas.[162]
On 22 July 2011, a report described the discovery of a gigantic cloud of water vapor containing “140 trillion times more water than all of Earth’s oceans combined” around a quasar located 12 billion light years from Earth. According to the researchers, the “discovery shows that water has been prevalent in the universe for nearly its entire existence”.[163][164]
Water has been detected in interstellar clouds within the Milky Way.[165] Water probably exists in abundance in other galaxies, too, because its components, hydrogen, and oxygen, are among the most abundant elements in the universe. Based on models of the formation and evolution of the Solar System and that of other star systems, most other planetary systems are likely to have similar ingredients.
Water vapor
Water is present as vapor in:
- Atmosphere of the Sun: in detectable trace amounts[166]
- Atmosphere of Mercury: 3.4%, and large amounts of water in Mercury’s exosphere[167]
- Atmosphere of Venus: 0.002%[168]
- Earth’s atmosphere: ≈0.40% over full atmosphere, typically 1–4% at surface; as well as that of the Moon in trace amounts[169]
- Atmosphere of Mars: 0.03%[170]
- Atmosphere of Ceres[171]
- Atmosphere of Jupiter: 0.0004%[172] – in ices only; and that of its moon Europa[173]
- Atmosphere of Saturn – in ices only; Enceladus: 91%[174] and Dione (exosphere)[citation needed]
- Atmosphere of Uranus – in trace amounts below 50 bar
- Atmosphere of Neptune – found in the deeper layers[175]
- Extrasolar planet atmospheres: including those of HD 189733 b[176] and HD 209458 b,[177] Tau Boötis b,[178] HAT-P-11b,[179][180] XO-1b, WASP-12b, WASP-17b, and WASP-19b.[181]
- Stellar atmospheres: not limited to cooler stars and even detected in giant hot stars such as Betelgeuse, Mu Cephei, Antares and Arcturus.[180][182]
- Circumstellar disks: including those of more than half of T Tauri stars such as AA Tauri[180] as well as TW Hydrae,[183][184] IRC +10216[185] and APM 08279+5255,[163][164] VY Canis Majoris and S Persei.[182]
Liquid water
Liquid water is present on Earth, covering 71% of its surface.[22] Liquid water is also occasionally present in small amounts on Mars.[186] Scientists believe liquid water is present in the Saturnian moons of Enceladus, as a 10-kilometre thick ocean approximately 30–40 kilometres below Enceladus’ south polar surface,[187][188] and Titan, as a subsurface layer, possibly mixed with ammonia.[189] Jupiter’s moon Europa has surface characteristics which suggest a subsurface liquid water ocean.[190] Liquid water may also exist on Jupiter’s moon Ganymede as a layer sandwiched between high pressure ice and rock.[191]
Water ice
Water is present as ice on:

- Mars: under the regolith and at the poles.[192][193]
- Earth–Moon system: mainly as ice sheets on Earth and in Lunar craters and volcanic rocks[194] NASA reported the detection of water molecules by NASA’s Moon Mineralogy Mapper aboard the Indian Space Research Organization’s Chandrayaan-1 spacecraft in September 2009.[195]
- Ceres[196][197][198]
- Jupiter’s moons: Europa‘s surface and also that of Ganymede[199] and Callisto[200][201]
- Saturn: in the planet’s ring system[202] and on the surface and mantle of Titan[203] and Enceladus[204]
- Pluto–Charon system[202]
- Comets[205][206] and other related Kuiper belt and Oort cloud objects[207]
And is also likely present on:
Exotic forms
Water and other volatiles probably comprise much of the internal structures of Uranus and Neptune and the water in the deeper layers may be in the form of ionic water in which the molecules break down into a soup of hydrogen and oxygen ions, and deeper still as superionic water in which the oxygen crystallizes, but the hydrogen ions float about freely within the oxygen lattice.[210]
Water and planetary habitability
The existence of liquid water, and to a lesser extent its gaseous and solid forms, on Earth are vital to the existence of life on Earth as we know it. The Earth is located in the habitable zone of the Solar System; if it were slightly closer to or farther from the Sun (about 5%, or about 8 million kilometers), the conditions which allow the three forms to be present simultaneously would be far less likely to exist.[211][212]
Earth’s gravity allows it to hold an atmosphere. Water vapor and carbon dioxide in the atmosphere provide a temperature buffer (greenhouse effect) which helps maintain a relatively steady surface temperature. If Earth were smaller, a thinner atmosphere would allow temperature extremes, thus preventing the accumulation of water except in polar ice caps (as on Mars).[citation needed]
The surface temperature of Earth has been relatively constant through geologic time despite varying levels of incoming solar radiation (insolation), indicating that a dynamic process governs Earth’s temperature via a combination of greenhouse gases and surface or atmospheric albedo. This proposal is known as the Gaia hypothesis.[citation needed]
The state of water on a planet depends on ambient pressure, which is determined by the planet’s gravity. If a planet is sufficiently massive, the water on it may be solid even at high temperatures, because of the high pressure caused by gravity, as it was observed on exoplanets Gliese 436 b[213] and GJ 1214 b.[214]
Law, politics, and crisis
This section needs to be updated. Please help update this article to reflect recent events or newly available information. (June 2022) |

Water politics is politics affected by water and water resources. Water, particularly fresh water, is a strategic resource across the world and an important element in many political conflicts. It causes health impacts and damage to biodiversity.
Access to safe drinking water has improved over the last decades in almost every part of the world, but approximately one billion people still lack access to safe water and over 2.5 billion lack access to adequate sanitation.[215] However, some observers have estimated that by 2025 more than half of the world population will be facing water-based vulnerability.[216] A report, issued in November 2009, suggests that by 2030, in some developing regions of the world, water demand will exceed supply by 50%.[217]
1.6 billion people have gained access to a safe water source since 1990.[218] The proportion of people in developing countries with access to safe water is calculated to have improved from 30% in 1970[219] to 71% in 1990, 79% in 2000, and 84% in 2004.[215]
A 2006 United Nations report stated that “there is enough water for everyone”, but that access to it is hampered by mismanagement and corruption.[220] In addition, global initiatives to improve the efficiency of aid delivery, such as the Paris Declaration on Aid Effectiveness, have not been taken up by water sector donors as effectively as they have in education and health, potentially leaving multiple donors working on overlapping projects and recipient governments without empowerment to act.[221]
The authors of the 2007 Comprehensive Assessment of Water Management in Agriculture cited poor governance as one reason for some forms of water scarcity. Water governance is the set of formal and informal processes through which decisions related to water management are made. Good water governance is primarily about knowing what processes work best in a particular physical and socioeconomic context. Mistakes have sometimes been made by trying to apply ‘blueprints’ that work in the developed world to developing world locations and contexts. The Mekong river is one example; a review by the International Water Management Institute of policies in six countries that rely on the Mekong river for water found that thorough and transparent cost-benefit analyses and environmental impact assessments were rarely undertaken. They also discovered that Cambodia’s draft water law was much more complex than it needed to be.[222]
In 2004, the UK charity WaterAid reported that a child dies every 15 seconds from easily preventable water-related diseases; often this means lack of sewage disposal.[citation needed]
Since 2003, the UN World Water Development Report, produced by the UNESCO World Water Assessment Programme, has provided decision-makers with tools for developing sustainable water policies.[223] The 2023 report states that two billion people (26% of the population) do not have access to drinking water and 3.6 billion (46%) lack access to safely managed sanitation.[224] People in urban areas (2.4 billion) will face water scarcity by 2050.[223] Water scarcity has been described as endemic, due to overconsumption and pollution.[225] The report states that 10% of the world’s population lives in countries with high or critical water stress. Yet over the past 40 years, water consumption has increased by around 1% per year, and is expected to grow at the same rate until 2050. Since 2000, flooding in the tropics has quadrupled, while flooding in northern mid-latitudes has increased by a factor of 2.5.[226] The cost of these floods between 2000 and 2019 was 100,000 deaths and $650 million.[223]
Organizations concerned with water protection include the International Water Association (IWA), WaterAid, Water 1st, and the American Water Resources Association. The International Water Management Institute undertakes projects with the aim of using effective water management to reduce poverty. Water related conventions are United Nations Convention to Combat Desertification (UNCCD), International Convention for the Prevention of Pollution from Ships, United Nations Convention on the Law of the Sea and Ramsar Convention. World Day for Water takes place on 22 March[227] and World Oceans Day on 8 June.[228]
In culture
Religion

Water is considered a purifier in most religions. Faiths that incorporate ritual washing (ablution) include Christianity,[229] Hinduism, Islam, Judaism, the Rastafari movement, Shinto, Taoism, and Wicca. Immersion (or aspersion or affusion) of a person in water is a central Sacrament of Christianity (where it is called baptism); it is also a part of the practice of other religions, including Islam (Ghusl), Judaism (mikvah) and Sikhism (Amrit Sanskar). In addition, a ritual bath in pure water is performed for the dead in many religions including Islam and Judaism. In Islam, the five daily prayers can be done in most cases after washing certain parts of the body using clean water (wudu), unless water is unavailable (see Tayammum). In Shinto, water is used in almost all rituals to cleanse a person or an area (e.g., in the ritual of misogi).
In Christianity, holy water is water that has been sanctified by a priest for the purpose of baptism, the blessing of persons, places, and objects, or as a means of repelling evil.[230][231]
In Zoroastrianism, water (āb) is respected as the source of life.[232]
Philosophy

The Ancient Greek philosopher Empedocles saw water as one of the four classical elements (along with fire, earth, and air), and regarded it as an ylem, or basic substance of the universe. Thales, whom Aristotle portrayed as an astronomer and an engineer, theorized that the earth, which is denser than water, emerged from the water. Thales, a monist, believed further that all things are made from water. Plato believed that the shape of water is an icosahedron – flowing easily compared to the cube-shaped earth.[233]
The theory of the four bodily humors associated water with phlegm, as being cold and moist. The classical element of water was also one of the five elements in traditional Chinese philosophy (along with earth, fire, wood, and metal).
Some traditional and popular Asian philosophical systems take water as a role-model. James Legge‘s 1891 translation of the Dao De Jing states, “The highest excellence is like (that of) water. The excellence of water appears in its benefiting all things, and in its occupying, without striving (to the contrary), the low place which all men dislike. Hence (its way) is near to (that of) the Tao” and “There is nothing in the world more soft and weak than water, and yet for attacking things that are firm and strong there is nothing that can take precedence of it—for there is nothing (so effectual) for which it can be changed.”[234] Guanzi in the “Shui di” 水地 chapter further elaborates on the symbolism of water, proclaiming that “man is water” and attributing natural qualities of the people of different Chinese regions to the character of local water resources.[235]
Folklore
“Living water” features in Germanic and Slavic folktales as a means of bringing the dead back to life. Note the Grimm fairy-tale (“The Water of Life“) and the Russian dichotomy of living [ru] and dead water [ru]. The Fountain of Youth represents a related concept of magical waters allegedly preventing aging.
Art and activism
Painter and activist Fredericka Foster curated The Value of Water, at the Cathedral of St. John the Divine in New York City,[236] which anchored a year-long initiative by the Cathedral on our dependence on water.[237][238] The largest exhibition to ever appear at the Cathedral,[239] it featured over forty artists, including Jenny Holzer, Robert Longo, Mark Rothko, William Kentridge, April Gornik, Kiki Smith, Pat Steir, Alice Dalton Brown, Teresita Fernandez and Bill Viola.[240][241] Foster created Think About Water,[242][full citation needed] an ecological collective of artists who use water as their subject or medium. Members include Basia Irland,[243][full citation needed] Aviva Rahmani, Betsy Damon, Diane Burko, Leila Daw, Stacy Levy, Charlotte Coté,[244] Meridel Rubenstein, and Anna Macleod.
To mark the 10th anniversary of access to water and sanitation being declared a human right by the UN, the charity WaterAid commissioned ten visual artists to show the impact of clean water on people’s lives.[245][246]
Dihydrogen monoxide parody
Water’s technically correct but rarely used chemical name, dihydrogen monoxide, has been used in a series of hoaxes and pranks that mock scientific illiteracy. This began in 1983, when an April Fools’ Day article appeared in a newspaper in Durand, Michigan. The false story consisted of safety concerns about the substance.[247]
Music
The word “Water” has been used by many Florida based rappers as a sort of catchphrase or adlib. Rappers who have done this include BLP Kosher and Ski Mask the Slump God.[248] To go even further some rappers have made whole songs dedicated to the water in Florida, such as the 2023 Danny Towers song “Florida Water”.[249] Others have made whole songs dedicated to water as a whole, such as XXXTentacion, and Ski Mask the Slump God with their hit song “H2O”.
See also
- Outline of water – Overview of and topical guide to water
- Water (data page) – Chemical data page for water is a collection of the chemical and physical properties of water.
- Aquaphobia – Persistent and abnormal fear of water
- Human right to water and sanitation – Human right recognized by the United Nations General Assembly in 2010
- Hydroelectricity – Electricity generated by hydropower
- Marine current power – Extraction of power from ocean currents
- Marine energy – Energy stored in the waters of oceans
- Mpemba effect – Natural phenomenon that hot water freezes faster than cold
- Oral rehydration therapy – Type of fluid replacement used to prevent and treat dehydration
- Osmotic power – Energy available from the difference in the salt concentration between seawater and river water
- Properties of water – Physical and chemical properties of pure water
- Thirst – Craving for potable fluids experienced by animals
- Tidal power – Technology to convert the energy from tides into useful forms of power
- Water pinch analysis
- Wave power – Transport of energy by wind waves, and the capture of that energy to do useful work
- Catchwater – Runoff catching or channeling device
PROCESS OF WATER TREATMENT

- This book has been formed from various articles available in our library and due acknowledge is given to respective authors and companies.
- The views expressed in the Articles are for general guidance only and no liability is accepted whatsoever arising out of its use.
- Typographical mistakes are not intentional may kindly be excused
INDEX ABOUT WATER TREATMENT :-
PARTICULARS
Page No
Introduction
4
How Reverse Osmosis Works
5
Understanding Total Dissolved Solids
6
General understanding of RO Membrane Maintenance and Cleaning
8
Understanding Reverse Osmosis
14
How to use Reverse Osmosis in Practice
16
Pretreatment required for RO
18
Pore Size recommended for Filter Cartridge
19
Reverse Osmosis Module Designs
20
Comparisons of Reverse Osmosis System Types
21
Estimated Percent Rejection of Various Solutes
23
Factors affecting RO Membrane Performance
25
Factors influencing Reverse Osmosis Performance
32
Handling & Preservation
34
Basics of RO & NF : Element Construction
36
Degasifier use for RO
38
RO Maintenance
39
RO Care
40
Preventive Care
41
Routine Maintenance
42
When to Clean an RO Plant
44
Reversing Problems in Reverse Osmosis
45
When to Clean
47
Chemical Attack
49
Changing Reverse Osmosis Membranes
50
Common Impurities in Water & Methods to remove it
51
Water Analysis conversion Table for Units Employed Equivalents
54
Indian Standard grade form the commonly used regeneration chemicals
56
Conversion factors for conversion to Calcium Carbonate
57
Filter Operation
59
Thumb rules for designing a filter
60
Important points on filter
61
Ion Exchange Operation
62
Softeners
65
BIS Standards for drinking water
71
Indian Standard for feed water & Boiler Water Limits
74
Hundred Ways to Save Water
77
Rain Water Harvesting
85
Introduction
We all eat in restaurants, get our vehicles washed, use laundries, see doctors in medical clinics or hospitals, attend or have children in schools, and patronize health and beauty spas and salons. Most of these facilities need water treatment.
While dealing with water management, one often finds that the source and the point of application are miles apart. Therefore, transporting water from one location to another plays a critical role in any industry. Water in its various forms (like raw water, treated water, sea water, wastewater, polluted water and sludge), along with various chemicals (like acid, alkali, lime, alum, polyelectrolyte, chlorine solution and salt solution) needs to be handled carefully while transportation.
Rapid industrialization, unplanned resource utilization and poor environment management have affected environmental resources resulting in deterioration of the quality of water. With the ever increasing TDS content in raw water and treated effluent, Reverse Osmosis (RO) has been emerged as most economical technology not only for the desalination of water containing salts, but also for eliminating heavy metals, pesticides etc.
How Reverse Osmosis Works
The phenomenon of osmosis occurs when pure water flows from a dilute saline solution through a membrane into a higher concentrated saline solution.
The phenomenon of osmosis is illustrated in Figure 1. A semi permeable membrane is placed between two compartments. “Semi permeable” means that the membrane is permeable to some species, and not permeable to others. Assume that this membrane is permeable to water, but not to salt. Then, place a salt solution in one compartment and pure water in the other compartment. The membrane will allow water to permeate through it to either side. But salt cannot pass through the membrane.
As a fundamental rule of nature, this system will try to reach equilibrium. That is, will try to reach the same concentration on both sides of the membrane. The only possible way to reach equillibrium is for water to pass from the pure water compartment to the salt-containing compartment, to dilute the salt solution.
Figure 1 also shows that osmosis can cause a rise in the height of the salt solution. This height will increase until the pressure of the column of water (salt solution) is so high that the force of this water column stops the water flow. The equilibrium point of this water column height in terms of water pressure against the membrane is called osmotic pressure.
If a force is applied to this column of water, the direction of water flow through the membrane can be reversed. This is the basis of the term reverse osmosis. Note that this reversed flow produces pure water from the salt solution, since the membrane is not permeable to salt.

- Understanding Total Dissolved Solids
Total Dissolved Solids (TDS) consist mainly of carbonates, bicarbonates, chlorides, sulphates, phosphates, nitrates, calcium, magnesium, sodium, potassium, iron, manganese, and a few others. They do not include gases, colloids, or sediment. The TDS can be estimated by measuring the specific conductance of the water. Dissolved solids in natural water range from less than 10 mg/L for rain to more than 100,000 mg/L for brines. Since TDS is the sum of all materials dissolved in the water, it has many different mineral sources. The chart below indicates the TDS from various sources.
Total Dissolved Solid (Mg/Ltr)
Distilled Water (0)
Two-column Deionizer Water (8)
Rain and Snow (10)
Ganges River Water (upto 10 km from start-30)
Average rivers in the U.S. (210)
Missouri River(360)
Pecos River(2600)
Oceans(35000)
Brine Well (125000)
Dead Sea (250000)
Yamuna River-Delhi (350)
High levels of total dissolved solids can adversely affect industrial applications requiring the use of water such as cooling tower operations, boiler feed water, food and beverage industries, and electronics manufacturers. High levels of chloride and sulphate will accelerate corrosion of metals. The US EPA has a suggested level of 500 mg/I listed in the Secondary Drinking Water Standards.
General Understanding of RO Membrane Maintenance and Cleaning
Following gives you a clear understanding of Membrane working and effectiveness of maintenance and cleaning. If practice is adopted and seriousness is developed membrane can give you consistent flow of required specified quality water apart from longer life.
One RO design feature that is commonly over looked in reducing RO cleaning frequency is the use of RO permeate water for flushing foul ants from the system. Soaking the RO elements during standby with permeate can help dissolve scale and loosen precipitates, reducing the frequency of chemical cleaning.
Eventually the day comes when your RO system will require cleaning. Cleaning is recommended when your RO shows evidence of fouling, just prior to a long-term shutdown, or as a matter of scheduled routine maintenance. Fouling characteristics that signal that you need to clean are- a 10-15% decrease in normalized permeate flow, a 10-15% decrease in normalized permeate quality, or a 10-15% increase in normalized pressure drop as measured between the feed and concentrate headers.
RO cleaning frequency due to fouling will vary by site. A rough rule of thumb as to an acceptable cleaning frequency is once every 3 to 12 months. If you have to clean more than once a month, you should be able to justify further capital expenditures for improved RO pre-treatment or a re-design of the RO operation. If cleaning frequency is every one to three months, you may want to focus on improving the operation of your existing equipment but further capital expenditure may be harder to justify.
What you clean for can vary site depending on the foulant. Complicating the situation frequently is that one more than one foulant can be present. Typical foul ants are:
- Calcium carbonate scale
- Sulphate scale of calcium, barium or strontium
- Metal oxides of iron, manganese, aluminum, etc.
- Silica scale
- Colloidal deposits (inorganic or mixed inorganic/organic)
- Organic material of natural origin or man-made origin
- Biological (bios lime, mold, or fungi)
There are a number of factors involved in the selection of a suitable cleaning chemical (or chemicals) and proper cleaning protocol. The first time you have to perform a cleaning, it is recommended to contact the manufacturer of the equipment, the RO element manufacturer, or a RO specialty chemical supplier. Once the suspected foulant(s) are identified, one or more cleaning chemicals will be recommended. These chemical(s) can be generic and available from a number of suppliers or can be private-labeled proprietary cleaning solutions. The proprietary solutions can be more expensive but may be easier to use and you cannot rule out the advantage of the intellectual knowledge supplied by these companies. An invaluable service offered some service companies pull that they will determine the proper cleaning chemicals and protocol by testing at their facility an element from your system.
It is not unusual to have to use a number of different cleaning chemicals in a specific sequence to achieve the optimum cleaning. There are times that a low pH cleaning is used first to remove foul ants like mineral scale, followed by a low pH cleaning. Some cleaning solutions have detergents added to aid in the removal of heavy biological and organic debris, while others have a chelating agent like EDTA added to aid in the removal of colloidal material, organic and biological material, and sulphate scale. An important thing to remember is that the improper selection of a cleaning chemical or the sequence of chemical introduction can make the foulant worse.
There are a number of precautions in cleaning chemical selection and usage for a composite polyamide membrane:
- Follow the manufacture’s recommended chemical list, dosage, pH, temperature and contact time guidelines.
- Use the least harshest chemical cleaning to get the job done. This will optimize the useful life
- Be prudent in the adjustment of pH at the low and high pH range to extend the useful life of the membrane. A “gentle” pH range is 4 to 10, while the harshest is 2 to 12.
- Don’t mix acids with caustics. Thoroughly rinse the 1st cleaning solution from the system before introducing the next solution.
- Flush out detergents with high pH permeates.
- Verify that proper disposal requirements for the cleaning solution are followed.
If your system has been fouled biologically, you may want to consider the extra step of introducing a sanitizing biocide chemical after a successful cleaning. Biocides can be introduced immediately after cleaning. Biocides can be introduced immediately after cleaning, periodically (e.g.once a week), or continuously during service. You must be sure however that the biocide is compatible with the membrane, does not create any healthy risks, is effective in controlling biological activity, and is not cost prohibitive.
The successful cleaning of RO on-site requires a well-designed RO cleaning skid. Normally this skid is not hard piped to the RO skid and uses temporary hosing for connections. It is recommended to clean a multi-stage RO one stage at a time to optimize cross flow cleaning velocity. The source water for chemical solution and rinsing should be RO permeate, DI water or at least soft water. Components must be corrosion proof. Major cleaning system components are:
- RO Cleaning Tank: This tank needs to be sized properly to accommodate the displacement of water in the hose, piping, and pressure vessels. The tank should be designed to allow 100% drainage, easy access for chemical introduction and mixing, a recirculation line from the RO Cleaning Pump, proper venting, overflow, and a return line located near the bottom to minimize foam formation when using a surfactant.
- RO Cleaning Pump: This pump needs to be sized to develop the proper cross flow velocity to scrub the membrane clean. The cleaning rate for a 8-inch diameter vessel is 30 to 40 gpm and for a 4-inch diameter vessel is 8 to 10 The maximum recommended pressure is 60 psi to minimize the production of permeate during cleaning and reduce the convective redeposition of foulant back on to the membrane surface.
- RO Cleaning Cartridge Filter: Normally 5 to 10-micron and is designed to remove foul ants that have been displaced from the cleaning process.
- RO Tank Heater or Cooler: The optimal temperature for cleaning is 35 to 45o One cannot forget that heat is generated and imparted by the RO Cleaning Pump during recirculation.
- RO Tank Mixer: This is recommended to get optimal mixing of chemical, though some designers rely solely on the slow introduction of chemical while maintaining a recirculation through the RO Cleaning Pump back to the tank.
- Instrumentation: Cleaning system instrumentation should be included to monitor flow, temperature, pressure, and tank level.
- Sample Points: Sample valves should be located to allow pH and TDS measurements off the RO Cleaning Pump discharge and the concentrate side recirculation return line.
- Permeate Return Line: A small amount of the cleaning solution can permeate through the membranes and so, a permeate side return line is recommended.
RO Cleaning procedures may vary dependent on the situation. The time required to clean a stage can take from 4 to 8 hours. The basic steps of cleaning are:
- Perform a low pressure flush with feed or permeate water to remove service concentrate and foul ants.
- Make the cleaning solution as per the manufacturer’s instructions.
- Introduce the cleaning solution to the first stage for 60 minutes. You may want to throttle the flow up slowly to minimize the plugging of the feed path with dislodged foulant. Send the displaced water and up to 20% of the fouled cleaning solution to drain before returning the cleaning solution back to the RO Cleaning Tank. Readjust the pH to the target when it changes more than 0.5 pH units.
- An optional soak and recirculation sequence can be used. The soak time can be from 1 hour to overnight depending on the manufacturer’s recommendations, but the cautions that the proper temperature and pH be maintained and that this does increase the chemical exposure time of the membrane.
- A low pressure Cleaning Rinse with permeate water is required to remove all traces of chemical from the Cleaning Skid and the RO Skid.
- Once all the stages of a train are cleaned, the RO can be placed back into service. It is not unusual for it to take from a few hours to a few days for the RO permeate quality to stabilize, especially after high pH cleanings.
It is exciting to have a successful cleaning and watch your pressures and permeate quality improve. On the flip side it is frustrating to have an unsuccessful cleaning. If the cleaning did not provide the results you were hoping for, you may want to consider talking to those suppliers who offer off-site services rather than proceed with a trial-and-error approach on site. Pull one or two elements from the front or back end and send them to a service company. A service company can determine the optimal cleaning procedure and also report how effective the cleaning was in restoring flow and salt rejection.

Understanding Reverse Osmosis
Semi permeable Membranes are the Heart of RO Systems
The process of reverse osmosis (RO) represents the finest level of liquid filtration available today. While ordinary liquid filters use a screen to separate particles from water streams, an RO system employs a semi permeable membrane that separates an extremely high percentage of unwanted molecules. For example, the membrane may be permeable to water molecules, but not to molecules of dissolved salt. If this membrane is placed between two compartments in a container as shown in Figure 1, and a salt solution is placed in one half of the container and pure water in the other, water passes through the membrane while the salt cannot.
Pressure is applied to Reverse Natural Osmotic Flow
Now a fundamental scientific principle comes into play. That is, dissimilar liquid systems will try to reach the same concentration of materials on both sides of the membrane. The only way for this to happen in our example, is for pure water to pass through the membrane to the salt-water side in an attempt to dilute the salt solution. This attempt to reach equilibrium is called osmosis. But if the goal in our example water purification system is to remove the salt from water, it is necessary to reverse the natural osmotic flow by forcing the salt water through the membrane in the reverse direction. This can be accomplished by applying pressure to the salt water as it’s fed into the system, creating a condition know as “reverse osmosis.”( See Figure 1).
Cross-flow Filtration Permits Long-term Performance
While the principals of reverse osmosis are simple, in practical terms, the RO process cannot go on indefinitely unless steps are taken to ensure that the membrane doesn’t become clogged by precipitated salts and other impurities forced against it by the pressurized stream of feed water. To significantly reduce the rate of membrane fouling, RO systems employ cross-flow filtration (shown in Figure 2), which allows water to pass through the membrane while the separate flow of concentrate sweeps rejected salts away from the membrane surface.
How to Use Reverse Osmosis In Practice
The simplified reverse osmosis process is shown in Figure 2.
With a high-pressure pump, pressurized saline feed water is continuously pumped to the ro system. In the system, consisting of a pressure vessel (housing) and a membrane element, the feed water will be split into a low saline product, called permeate and a high saline brine, called concentrate or reject. A flow-regulating valve, called concentrate valve, controls the percentage of feed water that is going to the concentrate stream and the permeated which will be obtained from the feed.
In the case of a spiral wound module consisting of a pressure vessel and several spiral wound elements, pressurized water flows into the vessel and through the channels between the spiral windings of the element. Up to seven elements are connected together within a pressure vessel. The feed water becomes more and more concentrated and will enter the next element, and at last exists from the last element to the concentrate valve where the applied pressure will be released. The permeate of each element will be collected in the common permeate tube installed in the center of each spiral wound element and flows to a permeate collecting pipe outside of the pressure vessels.
Pretreatment required for RO
Typical peratreatment consists of:
- Coarse filtration (~80 micron) to remove large materials
- Hypochloride addition to reduce biofouling potential
- Fine filtration using multimedia filters or clarification
- Bisulphide addition to reduce residual free chlorine
- Cartridge filter upstream of the feed pump and membranes
Additional pretreatment considerations: Waters with higher particle contents, measured by silt density index (SDI), require a higher degree of pretreatment to achieve acceptable quality. Systems using groundwater as the feed source frequently operate without hypochloride and bisulphide addition. Waters with high hardness may require softening and / or acid addition. Activated carbon may be needed for water with high organic content. The in-line addition of antiscalants may be required for waters with high scaling potential.
Pore Size recommended for Filter Cartridge in an RO
- Most commonly a filter with a micron rating of 5 micron absolute.
- With high levels of colloidal silica, lower micro-ratings are advisable.
Reverse Osmosis Module Designs
Four basic types of RO module designs are in commercial use: tubular, plate-and-frame, spiral wound, and hollow fibre modules.
The tubular and the plate-and-frame devices date back to the early days of RO membrane technology. Both of these designs involve a high initial capital cost and a low membrane packing density (very low for the tubular design). However, these designs can operate on highly fouling feed waters. Thus, these designs find use in the food industry (examples: milk concentration for cheese manufacture, tomato juice concentration), and in concentration/treatment of waste waters. They seldom compete with spirals and hollow fibre modules in desalination and water purification applications.
The design of spiral wound elements contains two layers of membrane glued back-to-back onto a permeate collector fabric (permeate channel spacer). This membrane envelope is wrapped around a perforated tube into which the permeate empties from the permeate channel spacer. Plastic netting is wound into the device, and maintains the feed-stream channel spacing. It also promotes mixing of the feed stream to minimize concentration polarization.
Comparisons of Reverse Osmosis System Types
|
The design of a hollow fiber permeator can package a tremendous amount of membrane area into a small volume. The difficulty in this approach, however, is that these fibers act almost like a string filter. This design requires a high level of feed water pretreatment to minimize the fouling potential of the feedwater. And when they are fouled, they are very difficult to regenerate by cleaning methods. Another aspect of hollow fiber permeators is that abrasion through fiber-fiber contact or via fiber contact with trapped particles appears to occur during RO operation. This results in gradual fall-off of salt rejection with time.
Above is a set of comparisons between the four basis module designs. Comparing their susceptibility to fouling for example, hollow fiber devices are much worse than spiral wound devices, which in turn are much worse than tubular devices and plate-and-frame devices.
Referring to system costs, spiral wound and hollow fiber systems are relatively equal on well water sources. For surface water sources, pretreatment costs tend to be higher for hollow fiber systems because of their fouling potential. Tubular and plate-and-frame systems are far more expensive than hollow fiber and spiral wound devices, and are relatively cost competitive to each other. As for system space requirements, tubular modules require the most space, hollow fiber and spiral modules require the least space.
One specific advantage of spiral wound units is that they can be linked together into series of two to seven elements within a single pressure vessel. Thus, up to seven times the flow of product water can be handled with only a single set of plumbing connections for feed, concentrate and permeate to a pressure vessel. In the case of hollow fiber modules, each hollow fiber unit requires installation of one feedwater inlet, one concentrate outlet, and one permeate outlet. For large modular systems for field application, a significant percentage of the system cost will be in the plumbing connections.
Estimated Percent Rejection of Various Solutes
Given below are the approximate Rejection percentages for various solutes
The data is based on the average taken from various manufacturers catalogs
Actual system performance may vary from the listed data, particularly with changes in feed water concentration, pH and temperature. For this reason, these tables should be used as a quick screen. Pilot trials should be performed to determine actual rejection in a specific application.
|
Solute |
MW |
Rejection, % |
|
Solute |
MW |
Rejection, % |
|
1,1,1-Trichloroethane |
133 |
97 |
Calcium Chloride |
111 |
99 |
|
|
1,2-Dibromoethane |
173 |
15 |
Calcium Nitrate |
164 |
95 |
|
|
1,2-Dichloroethane |
99 |
37 |
Carbon Tetrachloride |
153 |
98 |
|
|
1,2,3-Trichlorobenzene |
181 |
>57 |
Cesium Chloride |
168 |
97 |
|
|
1,2,4-Trichlorobenzene |
181 |
96 |
Chlorobenzene |
112 |
0-50 |
|
|
1,2,4-Trimethylbenzene |
120 |
57 |
Chloroform |
119 |
71-90 |
|
|
1,2-Dichlorobenzene |
147 |
70-92 |
Cis-1, 2-Dichloroethylene |
97 |
20 |
|
|
1,3-Dichlorobenzene |
147 |
66-69 |
Clofibric Acid |
214 |
>99 |
|
|
1,4-Dichlorobenzene |
147 |
61 |
Copper Sulfate |
160 |
99 |
|
|
1-Chlorododecane |
204 |
88 |
Cyclohexanone |
98 |
95 |
|
|
1-Methylnapthalene |
142 |
67 |
Dibromochloromethane |
208 |
79 |
|
|
2,2’,5,5’-Tetrachlorobiphenyl |
290 |
45 |
e-Caprolactum |
113 |
85 |
|
|
2,4,6-Trichlorophenol |
197 |
100 |
Ethanol |
46 |
38-70 |
|
|
2,4-Dichlorophenol |
163 |
93 |
Ethyl Benzene |
106 |
71 |
|
|
2,6-Dimethylphenol |
122 |
92 |
Formaldehyde |
30 |
35 |
|
|
2,6-DI-Tert-Butyl-4-Methylphenol |
220 |
96 |
Furfural |
96 |
35 |
|
|
3,8-Dimethylphenol |
122 |
92 |
Glucose |
180 |
98-99 |
|
|
3-Hydroxy-Capric Acid |
188 |
>98 |
Glycine |
188 |
78 |
|
|
3-Pentanone |
86 |
74 |
Heptaldehyde |
114 |
100 |
|
|
4-Ethylphenol |
122 |
84 |
Humic Acid |
98 |
||
|
4-Isopropylphenol |
136 |
84 |
Hydrochloric Acid |
36 |
28 |
|
|
5-Chlorouracil |
146 |
88 |
Isophorone |
138 |
96 |
|
|
Acetric Acid |
60 |
45 |
Isopropanol |
60 |
90 |
|
|
Acetone |
58 |
70 |
Lactic Acid (pH 2) |
90 |
94 |
|
|
Aluminium Nitrate |
213 |
86 |
Lactic Acid (pH 5) |
42 |
99 |
|
|
Aluminium Sulfate |
342 |
89 |
Magnesium Chloride |
120 |
98 |
|
|
Aniline |
93 |
64-75 |
Magnesium Sulfate |
120 |
99 |
|
|
Anthraquinone |
208 |
93 |
Manganese (II) Sulfate |
151 |
97 |
|
|
Benzene |
78 |
19 |
Methanol |
32 |
25 |
|
|
Benzoic Acid |
122 |
92 |
Methyl Ethyl Ketone |
72 |
73 |
|
|
Benzothiazole |
133 |
79 |
Methyl Isobutyl Ketone |
100 |
98 |
|
|
Biphenyl |
154 |
91 |
Napthalene |
128 |
80 |
|
|
Bis (2-Ethylhexyl) Pthalate |
390 |
94 |
Nickel Chloride |
130 |
96-99 |
|
|
Boric Acid |
230 |
Nickel Sulphate |
155 |
97-99 |
||
|
Bromodichloromethane |
163 |
79 |
o-Cresol |
108 |
84 |
|
|
Bromoform |
94 |
>67 |
o-Xylene |
106 |
67 |
|
|
Cadmium Sulfate |
208 |
97 |
p & m Xylene |
106 |
38 |
|
|
Caffeine |
174 |
99 |
Pentachlorophenol |
266 |
>86 |
|
|
Phenol-80% |
94 |
65 |
Sodium Orthophosphate |
164 |
99 |
|
|
Phosphoric Acid |
96 |
94 |
Stearic Acid |
204 |
71 |
|
|
Quinoline |
129 |
97 |
Strontium Chloride |
158 |
96 |
|
|
Silica |
60 |
98 |
Succinic Acid |
118 |
35 |
|
|
Sodium Acetate (1%) |
82 |
88 |
Sucrobe |
342 |
99 |
|
|
Sodium Bicarbonate |
84 |
98 |
Sulphuric Acid |
98 |
84 |
|
|
Sodium Bromide |
103 |
96 |
Tetrachloroethylene |
165 |
68-80 |
|
|
Sodium Chloride |
58 |
99 |
Tin(II) Sulphate |
215 |
85 |
|
|
Sodium Cyanide |
49 |
95 |
Tributyl Phosphate |
266 |
49 |
|
|
Sodium Di-H Phosphate |
120 |
98 |
Trichloroethylene |
131 |
30-43 |
|
|
Sodium Fluoride |
42 |
98 |
Trimesic Acid |
210 |
96 |
|
|
Sodium Hydrogen Sulfate |
12 |
76 |
Urea |
60 |
70 |
|
|
Sodium Iodide |
150 |
97 |
Zinc Chloride |
136 |
93 |
|
|
Sodium Mono-H Phosphate |
142 |
98 |
Zinc Sulfate |
161 |
98 |
|
|
Sodium Nitrate |
85 |
93-98 |
Factors Affecting RO Membrane Performance
Reverse osmosis (RO) technology can be a complicated subject, particularly without an understanding of the specific terminology that describes various aspects of RO system operation and the relationships between these operating variables.
This bulletin defines some of these key terms and provides a brief overview of the factors that affect the performance of RO membranes, including pressure, temperature, feed water salt concentration, permeate recovery, and system pH.
Definitions
Recovery – the percentage of membrane system feedwater that emerges from the system as product water or “permeate”. Membrane system design is based on expected feedwater quality and recovery is fixed through initial adjustment of valves on the concentrate stream. Recovery is often fixed at the highest level that maximizes permeate flow while preventing precipitation of super-saturated salts within the membrane system.
Rejection – the percentage of solids concentration removed from system feedwater by the membrane.
Passage – the opposite of “rejection”, passage is the percentage of dissolved constituents (contaminants) in the feedwater allowed to pass through the membrane.
Permeate – the purified product water produced by a membrane system.
Flow – Feed flow is the rate of feedwater introduced to the membrane element, usually measured in gallons per minute (gpm). Concentrate flow is the rate of flow of non-permeated feedwater that exists the membrane element. This concentrate contains most of the dissolved constituents originally carried into the element from the feed source. It is usually measures in gallons per minute (gpm).
Flux – the rate of permeate transported per unit of membrane area, usually measured in gallons per square foot per day (gfd).
Dilute solution – purified water solution, RO system product water.
Concentrated solution – brackish water solution such as RO system feedwater.
Effect of pressure
Feedwater pressure affects both the water flux and salt rejection of RO membranes. Osmosis is the flow of water across a membrane from the dilute side toward the concentrated solution side. Reverse osmosis technology involves application of pressure to the feedwater stream to overcome the natural osmotic pressure. Pressure in excess of the osmotic pressure is applied to the concentrated solution and the flow of water is reversed. A portion of the feedwater (concentrated solution) is forced through the membrane to emerge as purified product water of the dilute solution side
As shown in Figure 2, water flux across the membrane increases in direct relationship to increases in feedwater pressure. Increased feedwater pressure also results in increased salt rejection but, as Figure 2 demonstrates, the relationship is less direct than for water flux.
Because RO membranes are imperfect barriers to dissolved salts in feedwater, there is always some salt passage through the membrane. As feedwater pressure is increased, this salt passage is increasingly overcome as water is pushed through the membrane at a faster rate than salt can be transported.
However, there is an upped limit to the amount of salt that can be excluded via increasing feedwater pressure. As the plateau in the salt rejective curve (Figure 2) indicates, above a certain pressure level, salt rejection no longer increases and some salt flow remains coupled with water flowing through the membrane.
Effect of temperature As figure 3 demonstrates, membrane productivity is very sensitive to changes in feedwater temperature. As water temperature increases, water flux increases almost linearly, due primarily to the higher diffusion rate of water through the membrane.
Increased feedwater temperature also results in lower salt rejection or higher salt passage. This is due to a higher diffusion rate for salt through the membrane.
The ability of a membrane to tolerate elevated temperatures increases operating latitude and is also important during cleaning operations because it permits use of stronger, faster cleaning processes.
Effect of salt concentration Osmotic pressure is a function of the type and concentration of salts or organics contained in feedwater. As salt concentration increases, so does osmotic pressure. The amount of feedwater driving pressure necessary to reverse the natural direction of osmotic flow is, therefore, largely determined by the level of salts in the feedwater.
Figure 5 demonstrates that, if feed pressure remains constant, higher salt concentration results in lower membrane water flux. The increasing osmotic pressure offsets the feedwater driving pressure. Also illustrated in Figure 5 is the increase in salt passage through the membrane (decrease in rejection) as the water flux declines.
Effect of recovery As shown in Figure 1, reverse osmosis occurs when the natural osmotic flow between a dilute solution and a concentrated solution is reversed through application of feedwater pressure. If percentage recovery is increased (and feedwater pressure remains constant), the salts in the residual feed become more concentrated and the natural osmotic pressure will increase until it is as high as the applied feed pressure. This can negate the driving effect of feed pressure, slowing or halting the reverse osmosis process and causing permeate flux and salt rejection to decrease and even stop (please see Figure 6).
The maximum percent recovery possible in any RO system usually depends not on a limiting osmotic pressure, but on the concentration of salts present in the feedwater and their tendency to precipitate on the membrane surface as mineral scale. The most common sparingly soluble salts are calcium carbonate (limestone), calcium sulphate (gypsum), and silica. Chemical treatment of feedwater can be used to inhibit mineral scaling.
Effect of pH
The pH tolerance of various types of RO membranes can vary widely. Thin-film composite membranes are typically stable over a broader pH range than cellulose acetate (CA) membranes and, therefore, offer greater operating latitude (please see Figure 4).
Membrane salt rejection performance depends on pH. Water flux may also be affected. Figure 7 shows that water flux and salt rejection of a good membrane are essentially stable over a broad pH range.
As illustrated in Figure 4, the stability of membrane over a broad pH range permits stronger, faster, and more effective cleaning procedures to be used compared to CA membranes.
Factors Influencing Reverse Osmosis Performance
Permeate Flux and salt rejections are the key performance parameters of a reverse osmosis process. They are mainly influenced by variable parameters, which are as follows:
- Pressure
- Temperature
- Recovery
- Feed water salt concentration
The following graphs show the impact of each of those parameters when the other three parameters are kept constant. In practice, there is normally an overlap of two or more effects.
Not to be neglected are several main factors which cannot be seen directly in membrane performance. These are maintenance and operation of the plant as well as proper pretreatment design. Consideration of these three ‘parameters’, which have very strong impact on the performance of a reverse osmosis system, is a must for each OEM (original equipment manufacturer) and end user of such a system.
Pressure
With increasing effective feed pressure, the permeate TDS will decrease while the permeate flux will increase as shown in Figure 3.
Temperature
If the temperature increases and all other parameters and kept constant, the permeate flux and the salt passage will increase (see Figure 4).
Recovery
The recovery is the ratio of permeates flow to feed flow. In the case of increasing recovery, the permeate flux will decrease and stop if the salt concentration reaches a value where the osmotic pressure of the concentrate is as high as the applied feed pressure. The salt rejection will drop with increasing recover (see Figure 5).
Feedwater Salt Concentration
Figure 6 shows the impact of the feedwater salt concentration on the permeate flux and the salt rejection. Table 1 shows a summary of the impacts influencing reverse osmosis plant performance.
Handling and Preservation
Preservation Some new elements are sent in a standard preservation solution containing 1% sodium bisulphide. , Soaked in the mentioned solution for one hour, drained and bagged into a double plastic bag. The inner bag is made out of an oxygen barrier material.
Bisulphite provides protection from biological growth.
Some types of elements are also available as dry elements. Those elements have been dry vacuum tested, but not tested with saline water. They are bagged into a single plastic bag. They do not require any preservation solution, but they should be kept in their sealed bag until they are used.
Any element that has been used and removed from the pressure vessel for storage or shipping must be preserved in a preservation solution. Use a mixture of 1% (by weight) of sodium bisulfite – food grade, not cobalt activated – and water. Soak the element for one hour in the solution, allow dripping out and sealing it into an oxygen barrier plastic bag. Do not fill the plastic bag with the preservation solution – the moisture in the element is sufficient, and leaking bags might create a problem during transport. Identify the element and the preservation solution on the outside of the bag.
Rewetting of Dried Out Elements Elements that have dried out after use may irreversibly lose water permeability. Rewetting might be successful with one of the following methods:
- Soak in 50/50% ethanol/water or propanol/water for 25 min.
- Pressurize the element at 10 bar (150 psi) and close the permeate port for 30 min. Take care that the permeate port is reopened before the feed pressure is released.
- Soak the element in 1% HCI or 4% HNO3 for 11-30 hours.
Storage The following are guidelines for storage of elements :
- Store cool inside a building or warehouse and not in direct sunlight.
- Temperature limits: -4o C to + 35 o C (22 o F to 95 o F). New, dry elements will not be affected by temperatures below -4 o C (22 o F).
Elements stored in 1% sodium bisulphide will freeze below -4 o C (22 o F), Keep new elements in their original packaging.
Basics of RO and NF: Element Construction
Thin film composite membranes packed in a spiral wound configuration. Spiral wound designs offer many advantages compared to other module designs, such as tubular, plate and frame and hollow fiber module design for most of the reverse osmosis applications in water treatment. Typically, a spiral wound configuration offers significantly lower replacement costs, simpler plumbing systems, easier maintenance and greater design freedom than other configurations, making it the industry’s standard for reverse osmosis and nanofilteration(NF) membranes in water treatment.
The construction of a spiral wound membrane element as well as its installation in a pressure vessel is schematically shown in Figure 1.13. A element contains from one, to more than 30 membrane leafs, depending on the element diameter and element type. Each leaf is made of two membrane sheets glued together back-to-back with a permeate spacer in-between them. The consistent glue lines about 1.5 in (4 cm) wide that seal the inner (permeate) side of the leaf against the outer (feed/concentrate) side. There is a side glue line at the feed end and at the concentrate end of the element, and a closing glue line at the outer diameter of the element. The open side of the leaf is connected to and sealed against the perforated central part of the product water tube, which collects the permeate from all leaves. The leaves are rolled up with a sheet of feed spacer between each of them, which provides the channel for the feed and concentrate flow. In operation, the feed water enters the face of the element through the feed spacer channels and exists on the opposite end as concentrate. A part of the feed water – typically 10-20% – permeates through the membrane into the leaves and exists the permeate water tube.
When elements are used for high permeate production rates, the pressure drop of the permeate flow inside the leaves reduces the efficiency of the element.
In membrane systems the elements are placed in series inside of a pressure vessel. The concentrate of the first element becomes the feed to the second element and so on. The permeate tubes are connected with interconnectors (also called couplers), and the combined total permeate exits the pressure vessel at one side (sometimes at both sides) of the vessel.
Degasifier use for RO
Many people ask questionS regarding degasification. Typically degasification is not necessary. However, for specific needs, the use of a degasifier depends upon quality requirement needed and the composition of the feed water. Reasons to need a degasifier may be that a reduction of the CO2 or the H2S content is necessary. The use of a vacuum degasifer is preferable when H2S is present in the water or when airborne contaminations are present in the environment.
RO Maintenance
Understanding and reacting to the performance of a reverse osmosis (RO) system is necessary for continued successful operation. It is this interaction that allows us to quickly and correctly identify and correct issues that may arise. The following discussion is intended to explain the importance of RO maintenance.
First, we must understand why maintenance is needed. The following question will answer some of these needs.
- Have you ever had a problem with a RO unit?
- Have you ever had the same problem occur more than once?
- Has anyone ever asked about the performance before the problem occurred?
- Did anyone have documentation illustrating past performance?
Understanding and performing routine RO maintenance can prevent most problems before they occur. RO maintenance is more than repairing and replacing parts. It means taking steps to reduce or replacing parts. It means taking steps to reduce or prevent problems from occurring and being aware that a problem may be coming before it happens. Ensuring the RO is properly applied to the project and that feed water pretreatment (and the feed water itself) is checked on a regular basis also are instrumental. You also must check that normal scheduled maintenance occurs. If the system is large enough, daily log sheets are to be filled out. Maintenance is a combination of all these.
RO Care
An RO unit is only as good as the application allows. The first step in preventative care is to ensure the feed water is of satisfactory condition. Customers just don’t want to pay for that all-important feed water analysis, yet it cannot be stressed strongly enough. The larger the system, the greater the importance. Be aware of your feed water source. Surface water can produce needs that groundwater does not and vice versa. If your community mixes the two, it can be a “double whammy.” The point is to understand your feed water and install the proper pretreatment.
Understand both the amount of water and how the water will be used. Try to avoid traps such as knowing it needs to be 3,000 gallons per day and not knowing the day is eight hours. Make sure the unit is properly applied to the application and that any post treatment will allow proper flow and pressure.
Know the correct operating flows of the unit. Be sure to stay within manufacturer guidelines of pressure, product flow and recovery. Changes in feedwater temperature and total dissolved solids will change with the seasons. Expect these changes to cause minor adjustments to the unit.
Preventative Care
On initial installation, main plumbing lines are flushed and any pretreatment is properly working before you run water into the RO. Double check for the lack of carbon fines and water hardness. Carbon fines from improperly rinsed carbon beds or cartridges will cause premature failure of the 5-micron pre filter used for particle protection just prior to the RO. Hardness leakage may lead to fouling of the membranes. Once installed be certain to set the unit flows to those recommended by the RO manufacturer and put these settings on a start-up sheet. Instruct the operating personnel on the importance of proper operation. Ensure it is understood to keep a watch on any pretreatment and check it on a regular basis. For instance, if carbon filters are used, periodically test for chlorine.
Water softeners should be tested for hardness and/or iron leakage. Don’t test just the treated water; also test the incoming water. Remember the best time to test a water softener is just before it regenerates. Testing a freshly regenerated water softener (when regenerating properly) is of little use. Cities that have multiple sources of water will have varying amounts of constituents. The pretreatment must be capable of performing in the worst conditions. When checking pretreatment, be sure to check any regenerates such as salt in the brine tank. Do not let the brine tank run low or become empty. Salt on a skid next to a brine tank can cause problems.
Routine Maintenance
Regardless of system size certain tasks need to be performed on a regular basis. The most frequent maintenance is changing cartridge pre filters. These usually are nominally rated as 5 micron and are used to protect the RO membrane from particle fouling. Run length or time before changing is based on pressure drop. As these filters trap particles from the water supply, a reduction in pressure to the RO will occur. Most RO units include a low-pressure switch that prevents the RO from running if feed pressure drops too low. Check with your filter supplier to determine the allowable pressure drop across the cartridge and compare this to the incoming feed pressure. Applications with low feed pressure may not allow full use of these filters, requiring more frequent changes.
Carbon filters are commonly used for chlorine removal. Small systems may use carbon cartridges, while larger units may have backwashing carbon as well as other filter units. The time for carbon filter replacement is dependent on each application. Carbon cartridges should be replaced at least (if not before) every three months and backwashing filters should be changed annually if not before. Regardless of which type may exist, the change frequency is dependent on the application, size and type of cartridge and carbon as well as feed water make-up. Make certain the cartridges and any backwashing filters are well rinsed before sending any water to the RO. Backwashing filters should have an overnight presoak prior to use. It also is important to note that the use of some carbons is well-rinsed prior to placement online.
Check any RO pretreatment for correct operation. Check RO product water quality, system flows and pressure. Pressures include pre and post filters, RO pump discharge and waste and product water pressure. Keep a log sheet on this information and compare old data with new. Check the unit carefully for any leaks. Listen for any unusual noises. Pumps will exhibit a problem usually associated with a noise or leak prior to failure. Depending on size, the RO pump may be coupled to the motor and include an oil bowl reservoir. Be sure to check its level. Check to ensure that pressure switches and / or level controls are properly functioning.
Do not rush in and out. It is important to check the complete system both pre and post treatment. Make note of any deficiencies and take corrective action. Cleaning of the membranes usually is needed when the product flow rate falls 10 to 15 percent. When checking, it is necessary to ensure the loss of production is not caused by low feed water pressure, dirty pre filters or low feed water pressure. As a general rule, hardness scaling causes both a loss of water quality and flow rate. Biological fouling causes a loss of flow rate. If you are uncertain but believe a problem exists check with your RO supplier.
Data Log
Data log sheets are used to represent the past and present performance of an RO system. These sheets provide a window into expected future performance. Not all RO units need or have data logs. Some units are small enough that these sheets just do not make sense. You’ll know if your RO is large enough to need one of these. Just check the unit manual. If it needs one you will find it there. Typically, log sheets include all available operating data such as date, time, run time in hours, pre and post filter pressures, feed, concentrate and permeate pressures, feed water quality, permeate water quality, SDI, feed water hardness (ppm), chlorine, pH and others. The size and options selected will affect the data required for logging.
Use of these sheets allows the operator to spot trouble ahead of time. Through filling out these sheets, the operator will see patterns developing indicating normal or abnormal operation. Abnormal operation will indicate the type of problem that is occurring, allowing corrective action to take place. For example, if pressure drop rises, product water decreases and quality is falling, the need for cleaning is indicated. In this example, the need for an acid cleaning is indicated. In this example, the need for an acid cleaning to remove hardness scale is indicated. This is merely one example. A hardness fouling condition is described above for two reasons. The first one is that it indicates a problem is coming and how to correct it. The other is to illustrate that you now can go one step further. Hardness fouling indicates a possible failure in pretreatment or a change in raw water hardness level. This allows a correction of the problem and correction of the cause.
When to Clean an RO Plant
Generally it is recommended to clean an RO plant when a 10% decrease in normalized flux is observed. For orientation, cleaning frequency can be in the range of 4 year with an SDI of less than 3. With an SSDI of 5, the cleaning frequency could double. However, cleaning frequency will depend on the specific situation.
Reversing Problems in Reverse Osmosis
The useful life of reverse osmosis (RO) membrane elements is reduced by scaling, fouling and chemical attack. By preventing these processes from occurring, you can maximize membrane life.
Scaling results from the precipitation of certain feed water dissolved substances within an RO unit due to the concentration of feed water within the unit. The two most common scalants are calcium carbonate (limestone) and calcium sulphate (gypsum).
Scaling reduces permeate flow and increases permeate conductivity. Sharply pointed scale crystals may come into contact and cut the membrane, causing irreversible damage. Scalants other than polymerized silica generally will effectively be removed by chemically cleaning the affected elements with hydrochloric acid (muriatic acid) or citric acid. The pH of the cleaning solution should be no lower than the minimum pH allowed by the membrane manufacturer.
Polymerized silica scales are generally removed with a high pH cleaning solution. Caustic (sodium hydroxide) at the maximum pH allowed by the membrane manufacturer will remove polymerized silica scales. However, it will take many hours to remove a silica scale.
Most scaling can be eliminated by installing an upstream sodium-cycle ion exchange unit, commonly called sodium zeolite softeners. Silica scaling is less common, but more difficult to treat cost-effectively. An upstream dolomitic lime softener or the injection of a silica scale inhibitor will be required.
Fouling results when feedwater suspended particles are deposited within an RO unit. The most common fouling particles are bacteria, followed by aluminium, iron and silica.
Fouling reduces permeate flow rate. Bacterial fouling usually does not cause the permeate conductivity to increase until the system is extremely plugged. Other particles may cause the permeate conductivity to gradually increase. Sharp particles may cut the membrane and cause irreversible damage.
Most biological and particulate silica foulants can be removed y a high-pH detergent solution. The maximum pH is established by the membrane manufacturer. Appropriate and inappropriate detergents are specified by the membrane manufacturers. Aluminum and iron foulants are generally removed by scale-removing cleaning solutions.
Many vendors sell cleaning chemicals that are effective in cleaning RO membrane elements that can be used in place of the cleaning solutions described above or those recommended by the membrane manufacturers.
If biofouling is a problem, pretreatment may have little impact on RO unit fouling. For example, installing a UV (ultraviolet) unit upstream may have little effect on RO unit fouling. While the UV unit will kill or deactivate 90 to 100 percent of the feedwater bacteria, the same number of fouling particles (living and dead) enters the RO unit. The living bacteria can use the decomposing dead bacteria as food to promote swift re growth.
The action of chlorine, ozone, or other oxidizing compounds breaks down essential bacterial molecules, killing the bacteria or making it unable to reproduce. Additionally, these oxidizing compounds break down relatively large feedwater organic molecules that bacteria can’t eat, turning them into smaller, easily digested food molecules. The regrowth after chlorination/ dechlorination or after ozonation/deozonation may be very high.
Biofouling is usually more effectively handled by periodically cleaning and sanitizing the RO units and upstream equipment and piping that is not continuously chlorinated. Fouling by non-living particles is usually handled by installing sediment filters or cartridge filters with a nominal filtration rating of five micron or less.
When to Clean
Residential / commercial RO units typically are too small to justify the costs of installing instrumentation to determine when to chemically clean them. For a larger, industrial RO unit the following instruments are the minimum required to adequately monitor performance:
* Feedwater
Temperature, Conductivity, Pressure
* Interstage
Pressure
* Permeate
Conductivity, Flow, Pressure
* Concentrate
Conductivity, Flow, Pressure
Based upon the daily readings, graphs are generated that track normalized permeate flow (NPF), differential pressure (DP), and percent salt rejection (%SR). NPF tracks the pressures, conductivities, and temperature and determines if the amount of permeate produced is appropriate given the current conditions or whether too much pressure is required, which indicates whether scaling and /or fouling is present.
DPs are a measurement of the resistance to flow rate. A higher DP across one or more RO elements indicates the presence of a scalant and / or foulant.
Percent salt rejection measures the membrane’s ability to reject dissolved substances. Scaling and membrane damage cause the %SR to drop.
By monitoring the performance of an RO unit, the need for chemical cleaning becomes apparent. When the NPF drops by 10 to 15 percent, of if the DP across any stage increases by 10 to 25 percent, it is time to clean.
Cleaning at an early stage can remove most scalants and foulants. By waiting too long, however, the elements can become so plugged that channeling occurs. Small channels of high flow will develop in the elements. Cleaning solution will then go through the same channel as during normal service, but the plugged areas will remain plugged.
Again, for small residential / commercial units, instruments generally aren’t required to monitor performance. If you wait until the customer complains about taste, odor, and /or low – flow problems, the elements may be plugged significantly and a chemical cleaning may be ineffective or may take many hours to get the elements back to its almost – original performance. Periodic cleanings on a preventive maintenance contract may be worthwhile for certain customers.
Chemical Attack
Chlorine is the most common agent to chemically attack and destroy frequently used polyamide thin-film membranes. Activated carbon blocks are generally used upstream on smaller RO units to remove the chlorine. For larger units, dechlorination is accomplished either by an upstream granular AC (activated carbon) bed or by injecting a sulfite generating
(SO3-2) compound.
Active chlorine-consuming sites on the activated carbon material are depleted over time. Eventually dechlorination will diminish and finally quit, damaging downstream chlorine – sensitive membranes.
Activated Carbon blocks, or Activated Carbon beds, must be replaced as frequently as Activated Carbon manufacturers specify. However, if you periodically sanitize the Activated Carbon units with a chlorine solution or hydrogen peroxide (or other oxidant) solution, you’ll exhaust the Activated Carbon unit quicker than the design. Also, if the chlorine compound concentration in the feedwater increases, this will reduce the useful life of the Activated Carbon units. For example, it is common today for municipal drinking water treatment systems to periodically super-chlorinate the distribution system.
During most of the year, both gaseous chlorine and ammonia are commonly injected into municipal drinking water in order to provide microbiological control without creating excessive amounts of trihalomethane compounds. Since chloramines (chlorine plus ammonia) compounds are not as biocidal as free chlorine compounds, periodic super-chlorination with free chlorine residual is required to maintain microbiological control. If the expected useful life of an AC unit is based upon the usually low chloramines levels and doesn’t take into account a month or two per year of higher free chlorine residual, the AC unit may exhaust prematurely and cause downstream membrane damage.
Changing Reverse Osmosis Membranes
To open a PVC pressure vessel, first remove any pins holding one end plug in place. (Note: many fiberglass pressure vessels use a snap ring to hold the plug in place. Properly – sized snap ring pliers should be used to safely remove the snap ring). Next, remove the fittings from the plug.
Thread a nipple and tee into the feed or concentrate port long enough to extend past the end of the pressure vessel. Apply a coating of glycerin to the inside and rime of the pressure vessel from the plug to the end so the plug will slide out more easily. Pry off the plug using a pry bar. We either use a ball joint separator, also called a “pickle fork,” which is an automotive tool, or a slide hammer. Remove the fittings used to remove the plug.
Next, remove the membrane from the pressure vessel, noting which side the brine seal is no. Insert the new membrane with the brine seal on the feed side of the pressure vessel. Replace any damaged O-rings. Lubricate the plug with glycerin. Tap the plug securely into place using a piece of wood and a rubber mallet. Replace any pins to hold the plug in place. Reattach the fittings to the pin.
With a new membrane, flush all permeate and concentrate to drain for 30 minutes at low pressure to flush out the preservative.
Common Impurities in Water and Methods to Remove It
|
Impurities |
Effect |
Method of Removal |
|
Turbidity Suspended Silica |
Can clog pipelines and equipments can choke Ion exchange resin and RO membranes |
Coagulation, setting and filtration |
|
Color |
Indication of organic, iron, etc. and can be harmful to the unit operation ahead. |
Coagulation, setting filtration, followed by activated carbon filter. |
|
Organic matter |
Can foul Ion exchange resins membranes and may be detrimental to process. |
Coagulation, setting, filtration, followed by activated carbon filtration. |
|
Bacteria |
Will depend upon the type of bacteria, can induce corrosion and also harmful to RO membrane. |
Coagulation, filtration, setting and super chlorination, UV, ozonation |
|
Iron |
Red water, corrosion, deposit, interferes with dyeing, bleaching, etc. |
Aeration, coagulation, filtration, filtration through Manganese Zeolite |
|
PH |
High pH or low pH can both induce corrosion. |
Ion exchange, addition of acid or alkali. |
|
Calcium, Magnesium (Hardness) |
Scaling, cruds with soap interfere with dyeing and also harmful to other process. |
Ion exchange Lime Soda |
|
Sodium |
Unharmful when low in concentration, increase TDS, high concentration can induce corrosion |
Ion Exchange through cation H + resin. Reverse Osmosis |
|
Bicarbonates, Carbonates, Alkalinity, Hydroxide (Alkalinity) |
Corrosion, foaming and carry over. |
· Acid addition. · Ion Exchange by weak acid cation · Split stream by hydrogen cation resin · Degasification |
|
Sulphate |
Scaling if associated with Calcium, harmful in construction water. |
· Ion Exchange · Reverse Osmosis · Evaporation · Electrolysis. |
|
Chloride |
Corrosion |
· Ion Exchange · Reverse Osmosis · Evaporation · Electro dialysis |
|
Nitrate |
Normally not found in raw water. Harmful in food processes (especially baby food). |
· Ion Exchange · Reverse Osmosis |
|
Silica |
Scaling and deposition on equipment. |
Ion Exchange |
|
Carbon Dioxide |
Corrosion |
Open aeration, Degasification, and Vacuum deaeration. |
|
Hydrogen Sulphide |
Corrosion |
Aeration, filtration thorugh Manganese Zeolite, aeration plus chlorination. |
|
Oxygen |
Corrosion |
· Deaeration · Addition of chemicals likes sodium sulphite or hydrazine. · Anion exchanger |
|
Ammonia |
Corrosing especially of Copper and Zinc |
· Aeration · Hydrogenations Exchange if ammonia is present in Ionic form. |
|
Free Chlorine |
Corrosion |
· By adding chemicals · Activated Carbon |
Water Analysis Conversion Table for Units Employed: Equivalents
|
Water Analysis Conversion Table |
Parts per million (ppm) |
Milligrams per litre Mgm/l |
Grams per Litre gms/L |
Parts per hundred thousand pts/100000 |
Grains per U.S. gallons grs/U.Sgal |
Grains per british lmp gallon Grm/Impgal |
Kilograins per cubic foot Kgr/cu.ft |
|
1 Part per million (1 ppm) |
1 |
1 |
.001 |
.1 |
.0583 |
.07 |
.0004 |
|
1 milligram per litre (1mg/litre) |
1 |
1 |
.001 |
.1 |
.0583 |
.07 |
.0004 |
|
1 gram per litre (1 m/ litre) |
1000 |
1000 |
1 |
100 |
58.3 |
70 |
.435 |
|
1 Parts per hundred thousand (1pt/ 1000000) |
10 |
10 |
.01 |
1 |
.583 |
.7 |
.00436 |
|
1 Grain per U.S. gallon (1 gr/U.S gal) |
17.1 |
17.1 |
.017 |
1.71 |
1 |
1.2 |
.0075 |
|
1 Grain per british Imp gallon (1 gr/Imp gal) |
14.3 |
14.3 |
.014 |
1.43 |
.833 |
1 |
.0052 |
|
1 Kilograin per cubic foot (1 Kgr/cu.ft) |
2294 |
2294 |
2.294 |
229.4 |
134 |
161 |
1 |
|
Conversion Table |
Parts CaCO3 per million (ppm) |
Parts CaCO3 per hundred thousand (ft/100000 |
Grains CaCO3 per U.S. gal gpg |
English Degrees or CLARK |
French Degrees French |
German Degrees German |
Milli equivalents per litre or equivalents per million |
|
1 Parts per million (1 ppm) |
1 |
0.1 |
.0583 |
.07 |
.1 |
.0560 |
.020 |
|
1 Part per hundred thousand (1pt/100000) |
10 |
1 |
0.583 |
0.7 |
1 |
0.560 |
.20 |
|
1 Grain per Us gallon (1 gpg) |
17.1 |
1.71 |
1 |
1.2 |
1.71 |
0.958 |
.343 |
|
1 English or Clark degree |
14.3 |
1.43 |
.833 |
1 |
1.43 |
0.800 |
.286 |
|
1 French Degrees (1.French) |
10 |
1 |
.583 |
.7 |
1 |
0.560 |
20 |
|
1 German Degrees (1 German) |
17.9 |
1.79 |
1.04 |
1.24 |
1.79 |
1 |
.357 |
|
1 Milli equivalent / litre or 1 equivalent per million |
50 |
5 |
2.92 |
2.50 |
2.80 |
1 |
Indian standard grade form the commonly used regeneration chemicals
Hydrochloric Acid : IS 265
Sulphuric Acid : IS 266
Sodium Hydroxide : IS 252 (Tech/Rayon Grade 46% lys)
IS 1021 (Pure Grade – Flakes)
Sodium Carbonate : IS 251 (Tech Grade)
Sodium Suphite : IS 247 (Tech Grade)
Sodium Chloride : IS 297 (Tech Grade)
Alum : IS 260 (Tech Grade)
Conversion Factors for conversion to Calcium Carbonate (CACO3)
|
Ions |
Symbol |
Ionic weight |
Equivalent Weight |
To conver to to CaCO3 multiply by |
|
CATIONS |
||||
|
Aluminium |
Al+++ |
27.0 |
9.0 |
5.56 |
|
Ammonium |
NH4+ |
18.0 |
18.0 |
2.78 |
|
Barium |
Ba++ |
137.4 |
68.7 |
.728 |
|
Calcium |
Ca+ |
40.1 |
20.0 |
2.49 |
|
Copper |
Cu++ |
63.6 |
31.8 |
1.57 |
|
Hydrogen |
H+ |
1.0 |
1.0 |
50.0 |
|
Iron (Ferrous) |
Fe++ |
55.85 |
27.8 |
1.80 |
|
Iron (Ferric) |
Fe++ |
55.85 |
18.6 |
2.69 |
|
Magnesium |
Mg++ |
24.3 |
12.2 |
4.10 |
|
Manganese |
Mn++ |
54.9 |
27.5 |
1.82 |
|
Potassium |
K+ |
39.1 |
39.1 |
1.28 |
|
Sodium |
Na+ |
23.0 |
23.0 |
2.17 |
|
ANIONS |
||||
|
Bicarbonate |
HCO3- |
61.0 |
61.0 |
0.82 |
|
Bisulphate |
HSO4- |
97.1 |
97.1 |
0.515 |
|
Bisulphite |
HSO3- |
81.1 |
81.1 |
0.617 |
|
Carbonate |
CO3- |
60.0 |
30.0 |
1.67 |
|
Chloride |
Cl- |
35.5 |
35.5 |
1.41 |
|
Fluoride |
F- |
19.0 |
19.0 |
2.63 |
|
Hydroxide |
OH- |
17.0 |
17.0 |
2.94 |
|
Nitrate |
NO3- |
62.0 |
62.0 |
0.807 |
|
Phosphate (monovalent) |
H2PO4- |
97.0 |
97.0 |
0.516 |
|
Phosphate (divalent) |
HOP4- |
96.0 |
48.0 |
1.04 |
|
Phosphate (trivalent) |
PO4- |
95.0 |
31.7 |
1.58 |
|
Sulphate |
SO4- |
96.1 |
48.0 |
1.04 |
|
Sulphide |
S- |
32.1 |
16.0 |
3.12 |
|
Sulphite |
SO3- |
80.1 |
40.0 |
1.25 |
Filter Operation
Basic Operation of Filter : Basic operation of Pressure Filter, Dual Media Filter and Activated Carbon is as follows :
Mode of Operation : All units operate in down flow mode, where the water enters from the top, percolates through the media and treated water is collected from the bottom.
Sequence of Operation :
- Service : The water to be filtered enters from the top of the shell, percolates downward through the media and is drawn off from the bottom.
- Backwash : The water enters from the bottom of the vessel, passes through the media and is drained from the top. This is called BACKWASH and it is done to carry the dirt accumulated on the top. Generally back washing is done once in every 24 hrs or when the pressure drop exceeds 8 psi. (0.5 kg/cm2)
- Rinse : The water enters from the top passed through the media and is drained off from the bottom.
Thumb rules for designing a filter
Calculate areas of vessel by required volumetric flow rate and the velocity as mentioned in the following table :
Area (m2) = Volumetric Flow Rate (m3/hr)
Velocity (m/hr)
Based on above calculated area calculate dia of the vessel by the following formulae :
Dia (m) = (Area (m2)/0.7856)
|
Parameters |
Sand Filters |
Dual Media Filters |
Activated Carbon |
|
Velocity (m3/m2/hr) |
7.5-12 |
12.20 |
15.20 |
|
Effective size of Media (mm) |
0.45-0.6 (fine sand) |
0.65-0.76 (Anthracite) |
0.35-0.5 |
|
Uniform coefficient |
1.6 max |
1.85 |
< 2 (11.5 typical) |
|
Density (kg/m3) |
2650 |
1600 |
Important points on Filter:
- Normally, pressure sand filter is used to filter suspended solids upto 30 ppm and dual fitlers form 50-55 ppm and higher suspended solids would require coaulation. Output quality of water from a Pressure Sand Filter is 25 to 50 microns.
- Normally, velocity for Sand velocity is taken for water treatment / residential liters is taken as 7.5 to 18 m3/m2/hr for institutional filters 20 to 30 m3/m3/hr. For recirculation of water like swimming pool velocities can be taken greater than 35 m3/m2/hr. For recirculation of water like swimming pool velocities can be taken greater than 35 m3/m2/hr for low turbidity application.
- Higher velocity will induce higher head loss through the bed and frequency of backwash will increase.
- Back washing of filter should always be carried out using clean water.
- Whenever air scouring is provided, it should be done before back washing step.
- Where strainers are provided at bottom, pebblers and gravels need not be put.
- 1 NTU is approximately between 2.5 to 3 ppm.
Ion Exchange Operation
Ion Exchange Load Calculation
Let us take the following examples :
Feed water analysis as ppm CaCO3
Calcium – 210 Bicarbonate – 200
Magnesium – 40 Sulphate – 85
Sodium – 120 Chloride – 70
Potassium – 5 Nitrate – 20
——- ————
375 375
——- ————
Free CO2 – 15
Silica – 5
Note :
- Match total cations to total cations to total Anions. They should be equal. (Error of +- 5% can be considered)
- Refer to the table for calculating the Resin Quantity. The Ion Exchange load can be taken as mentioned in the table.
- For eg. For Softening : hardness is 250 ppm, therefore the Ion Exchange load is 250 pm.
|
|
Unit Operation |
Ion Exchange Load |
Concentration (as ppm CaCO3) |
|
Softening Dealkaization Strongly acid Cation Weakly basic Anion Strongly acid Cation after dealkalization Strongly Basic Anion after WBA Strongly Basic Anion after Degassing Strongly basis Anion after degassing and WBA Mixed Bed Resin (Mixture of Cotton / Anion) |
Total Hardness (Ca+Mg) HCO3 Total Cations (Ca+Mg+Na+K) EMA (SO4+Cl+NO3) Total Cations – Carbonate Hardness Total Anions – EMA Cl+SO4+NO3+SiO2 (Alkalinity + CO2) Total Anion-T.Alk-EMA + SiO2 Total electrolyte after SBA |
250 200 375 175 175 225 185(assuming 5ppm leakage of CO2) 10ppm (assuming 5ppm leakage) |
|
Parameters |
Cation |
Anion |
Mixed bed |
Degassifier |
|
Velocity* |
15-20 mtr/hr |
15-20 mtr/hr |
30-44 mtr/hr |
50-70 mtr/hr |
|
Bed Depth |
900-2000 mm |
900-2000mm |
1000-2000mm |
2400-3600mm |
|
Free Board |
60-100% |
60-100% |
60-100% |
|
|
Type of Interval |
Hub / radial Strain on plate |
Hub/ radial Strain on plate |
Hub/ radial Strain on plate |
Rashing rings Poll rings |
- Velocity is based on Ion Exchange resin and will varry from manufacturer to manufacturer resin.
- Free Board is required for down flow unit, for WAC resin the freed board should be 100%
- Softeners
Softening by ion exchange resin is the most common and probably the easiest method of removing hardness (that is calcium and magnesium) from water and render the water suitable for utility purpose. As the name implies ion exchange is a process in which undesirable ions are exchanged for more desirable ions.
Problems caused by Hard Water
Hard water does not lather easily with soap. This causes problems during washing and bathing and prevents proper cleaning. Hard water can also cause scaling in pipes, fittings and storage tanks. Scaling in geysers can increase the electricity consumption bills. Hard water is also bad for the skin and hair.
Softening Process Work
The softening process consists of passing raw water containing hardness through a bed of cation resin in sodium form. The hardness ions Ca & Mg are taken by resin and in exchange, the sodium ions are relinquished from the resin. This is called the service cycle.
Ca++ + 2A + Na2R à CaR + 2 Na+ + 2 A
Mg++ + 2A + Na2R à MgR + 2 Na+ + 2 A
Where A represents the relevant anions of bicarbonates, sulphates or chlorides R represents the anionic part of the softener resin
Regeneration of Softener
Raw water will continue to get softened till the resin gets exhausted. Bringing back the resin to it original form is called regeneration. Softener resin is regenerated by sodium chloride of 10-15% concentration. Depending on the softener design, the regeneration may need to be done every day or every few days or every week.
CaR + 2NaCl à Na2R + CaCl2
MgR + 2NaCl à Na2R + MgCl2
Softener Operation (Basic Ion Exchange Process)
Thumb rules of designing a Softener
Step 1 – To Select resin quantity (ltrs) for a particular hardness (ppm) for a particular output (m3) per regeneration per hour based on regeneration level 160 gm/ltr, ion exchange capacity = 55, TDS limit = 1500 ppm refer Table 1
Resin Quanitity = Load (ppm as CaCO3) * Flow * time
Ex. Capacity
For example
Load = Hardness = 100 ppm as CaCO3
Flow = 5M3/hr
Time = (Service Cycle) = 12 hrs.
Ex. Capacity =60 gm as CaCO3
Resin Quanitity = 100 * 5 * 12
= 60
100 litres
Note :
- Na/Tc and TDS and correction factor should be applied.
- Actual Resin Quantity = 60* correction due Na/Tc factor * Correction due to TH factor = 60 * 0.96 * 0.97 = 56 (approximately)
Hence Ion Exchange load for designing a softener is 56. These calculations are based on Ion Exchange resin and will varry from manufacturer to manufacturer resin.
Step 2 – To select vessel model for a selected resin quantity, approx. flow rates based on linear velocity min = 8 m3/m2/hr and max = 25 m3/m2/hr, and freeboard 5-100 %, refer Table 2
Important points on Softener
- Linear velocity should not be less than 8m3/m2/hr and should not be more than 25m3/m2/hr.
- Regeneration level, hardness leakage desired and correction factors can be found from resin supplier graph Tables for all these are shown but the actual resin supplier graph should be used. These tables can be used for first calculation.
- Suggested vessel selection chart for softeners
Table 1 : Step 1 – To select resin quantity (ltrs) for a particular hardness (ppm) for a particular output (m3) per regeneration per hour based on regeneration level160 gm/ltr. Ion exchange capacity = 55,TDS limit = 1500 ppm)
|
Hardness = 150ppm |
Hardness =250ppm |
Hardness = 350 ppm |
Hardness = 500 ppm |
Hardness = 650 ppm |
Hardness = 800 ppm |
Hardness = 1000 ppm |
|
|
5 |
13.5 |
22.5 |
31.5 |
45.0 |
58.5 |
72.0 |
90.0 |
|
10 |
27. |
45.0 |
63.0 |
90.0 |
117.0 |
144.0 |
180.0 |
|
15 |
39.0 |
65.0 |
91.0 |
1.35 |
175.5 |
216.0 |
270.0 |
|
20 |
52.5 |
87.5 |
122.5 |
180 |
234.0 |
288.0 |
360.0 |
|
25 |
66.0 |
110.0 |
154.0 |
225 |
292.5 |
360.0 |
450.0 |
|
30 |
79.5 |
132.5 |
111.3 |
270 |
351.0 |
432.0 |
540.0 |
|
35 |
91.5 |
152.5 |
213.5 |
315 |
409.5 |
504.0 |
630.0 |
|
40 |
105.0 |
175.0 |
245.0 |
360 |
468.0 |
576.0 |
720.0 |
|
45 |
118.5 |
197.5 |
276.5 |
405 |
526.5 |
648.0 |
810.0 |
|
50 |
132.0 |
220.0 |
308.0 |
450 |
585.0 |
720.0 |
900.0 |
|
55 |
144.0 |
240.0 |
336.0 |
495 |
643.5 |
792.0 |
990.0 |
|
60 |
157.2 |
262.0 |
366.8 |
540 |
702.0 |
864.0 |
1080.0 |
|
65 |
171.0 |
285.0 |
399.0 |
585 |
760.5 |
936.0 |
1170.0 |
|
70 |
184.5 |
307.5 |
430.5 |
630 |
819.0 |
1008.0 |
1260.0 |
|
75 |
198.0 |
330.0 |
462.0 |
675 |
877.5 |
1080.0 |
13500 |
|
80 |
210.0 |
350.0 |
490.0 |
720 |
936.0 |
1152.0 |
1440.0 |
|
85 |
223.0 |
372.0 |
521.0 |
765 |
994.5 |
1224.0 |
1530.0 |
|
90 |
237.0 |
395.0 |
553.0 |
810 |
1053.0 |
1296.0 |
1620.0 |
|
90 |
250.0 |
417.5 |
584.5 |
855 |
1111.5 |
1368.0 |
1710.0 |
|
100 |
262.5 |
437.5 |
612.5 |
900 |
1170.5 |
1440.0 |
1800.0 |
BIS Standards for Drinking Water
|
Characteristic |
IS Requirement Limit |
IS Permissible Limit |
WHO Guidelines |
US EPA Limit |
Essential Characteristics
|
Colour (in Hazen Units) |
5 |
25 |
15 |
15 |
|
Odour |
Unobjectionable |
– |
– |
– |
|
Taste |
Agreeable |
– |
– |
– |
|
Turbidity (in NTU) |
5 |
10 |
5 |
– |
|
PH |
6.5-8.5 |
6.5-8.5 |
– |
6.5-8.5 |
|
Total Hardness (as CaCO3) |
300 |
600 |
– |
– |
|
Iron (as Fe) |
0.3 |
1.0 |
0.3 |
0.3 |
|
Chlorides (as Cl) |
250 |
1000 |
250 |
250 |
|
Residual Free Chlorine |
0.2 |
– |
– |
– |
|
Dissolved Solids |
500 |
2000 |
1000 |
500 |
|
Calcium |
75 |
200 |
– |
– |
|
Copper |
0.05 |
1.5 |
2 |
1.3 |
|
Manganese |
0.1 |
0.3 |
0.5 |
0.05 |
|
Sulphate |
200 |
400 |
250 |
250 |
|
Nitrate |
45 |
100 |
50 |
10 |
|
Fluoride |
1.0 |
1.5 |
1.5 |
4 |
|
Phenolic Compounds |
0.001 |
0.002 |
– |
– |
|
Mercury |
0.001 |
0.001 |
0.001 |
0.002 |
|
Cadmium |
0.01 |
0.01 |
0.003 |
0.005 |
|
Selemium |
0.01 |
0.01 |
0.01 |
0.05 |
|
Arsenic |
0.05 |
0.05 |
0.01 |
– |
|
Cyanide |
0.05 |
0.05 |
0.07 |
0.2 |
|
Lead |
0.5 |
0.05 |
0.01 |
Zero |
|
Zinc |
5 |
15 |
3 |
5 |
|
Anionic Detergents |
0.2 |
1.0 |
– |
– |
|
Chromium |
0.05 |
0.05 |
0.05 |
0.1 |
|
Polynuclear aromatic hydrocarbons |
– |
– |
– |
– |
|
Mineral oil |
0.01 |
0.03 |
– |
– |
|
Pesticides |
Absent |
0.001 |
– |
– |
|
Alkalinity |
200 |
600 |
– |
– |
|
Aluminium |
0.03 |
0.2 |
0.2 |
0.05-0.2 |
|
Boron |
1 |
5 |
0.3 |
– |
|
Radioactive materials |
||||
|
a) Alpha emitters |
– |
0.1 |
0.1 |
None |
|
b)Beta emitters |
– |
1 |
1 |
None |
|
Upto 20 kg/cm2 |
21 to 39 kg/cm2 |
40 to 59 kg/cm2 |
IS35550 1965* |
IS3025 1965* |
|
|
Feed Water |
|||||
|
Total hardness (as CaCO3) mg/L Max |
10 |
1.0 |
0.5 |
16.1 |
|
|
pH Value |
8.5-9.5 |
8.5-9.5 |
8.5-9.5 |
– |
8 |
|
Dissolved Oxygen mg/L |
0.1 |
0.02 |
0.01 |
25 |
– |
|
Silica (As SiO2) mg/l Max |
– |
5 |
0.5 |
16 |
– |
|
Boiler Water |
|||||
|
Total Hardness (of filtered sample as CaCO3) as mg/L max |
– |
Not Detectable |
– |
– |
16.1 |
|
Total Alkalinity (As CaCO3) as mg/L max |
700 |
500 |
300 |
– |
13 |
|
Caustic Alkalinity (As CaCO3) mg/L Max |
350 |
200 |
60 |
– |
15 |
|
PH Value |
11-12 |
11-12 |
10.5-11 |
– |
8 |
|
Residual Sodium Sulphite (as Na2SO3) mg/l |
30-50 |
20-30 |
– |
– |
21 |
|
Residual Hydrazine (as N2H4) mg/l |
0.1-1 (if added) |
0.1-0.5 (if added) |
0.05-0.3 |
26 |
– |
|
Ration Na2SO4 Caustic Alkalinity (as NaOH) or Ration NaNO3 total alkalinity as NaOH |
– |
Above 2.5 |
– |
– |
20.2 & 15 |
|
Phosphates (PO4) mg/L (if added) |
20-40 |
15-30 |
5-20 |
14 |
– |
|
Total dissolved solids mg/L Max |
3500 |
2500 |
1500 |
9 |
12 |
|
Silica (as SiO2) Mg/L Max |
Less than 0.4 of caustic alkalinity |
15 |
16 |
30 |
All of us come in contact with water in all aspects of our lives, at homes, offices, offices, outdoors and at leisure. We all have that opportunity to save water at all those occasions. It is upto us to take that opportunity.
- When washing dishes, don’t let the water run while rinsing. Fill one sink with wash water and the other with rinse water.
- Evaporative coolers require a seasonal maintenance checkup. For more efficient cooling, check your evaporative cooler annually.
- Check your sprinkler system frequently and adjust sprinklers so only your lawn is watered and not the house sidewalk, or street.
- Run your washing machine and dishwasher only when they are full and you could save 1000 gallons a month.
- Avoid planting turf in areas that are hard to water such as steep inclines and isolated strips along sidewalks and driveways.
- Install covers on pools and spas and check for leaks around your pumps.
- Use the garbage disposal less often.
- Plant during the spring or fall when the watering requirements are lower.
- Keep a pitcher of water in the refrigerator instead of running the tap for cold drinks.
- Check your water meter and bill to track your water usage.
- Always water during the early morning hours, when temperatures
are cooler, to minimize evaporation.
- Wash your produce in the sink or a pan that is partially filled with
water instead of running water from the tap.
- Use a layer of organic much around plants to reduce evaporation
and save hundreds of gallons of water a year.
- Use a broom instead of a hose to clean your driveway and
sidewalk and save up to 80 gallons of water every time.
- If your shower can fill a one-gallon bucket in less than 20 seconds,
then replace it with a water-efficient showerhead
- Collect the water you use for rinsing produce and reuse it to water
houseplants.
- Water your lawn in several short sessions rather than one long
one. This will allow the water to be better absorbed.
- We’re more likely to notice leaky faucets indoors, but don’t forget
to check outdoor faucets, pipes, and hoses for leaks.
- Periodically check your pool for leaks if you have an automatic
refilling device.
- Only water your lawn when needed.
- While stopping a new appliance, keep in mind that one offering
several different cycles will be more water and energy-efficient.
- Time your shower to keep it under 5 minutes. You’ll save up to
1000 gallons a month.
- Install low-volume toilets.
- Adjust your lawn mower to a higher setting. Longer grass will
reduce the loss of water of evaporation.
- When you clean your fish tank, use the water you’ve drained on
your plants.
- Water small areas of grass by hand to avoid waste
- Put food coloring in your toilet tank. It’s easy to fix, and you can
save more than 600 gallons a month.
- Plug the bathtub before turning the water on, hten adjust the
temperature as the tub fills up.
- Use porous materials for walkways and patios to keep water in
your yard and prevent wasteful runoff.
- Collect and use rain water for watering your garden. (Check to
make sure this is legal in your area).
- Designate one glass for your drinking water each day. This will cut
down on the number of times you run your dishwater.
- Instead of using a hose or a sink to get rid of paints, oil and
pesticides, dispose of them properly to a hazardous waste site.
- Install rain shut-off device on your automatic sprinkles to
eliminate unnecessary watering.
- Don’t use running water to thraw food.
- Choose water-efficient drip irrigation for trees, shrubs, and
flowers, watering roots is very effective.
- Grab a wrench and fix that leaky faucet. It’s simple, inexpensive
and can save 140 gallons a week.
- Cut back on the amount of grace in your yard by planning shrubs
and ground cover or landscaping with rock.
- When doing laundry match the water level to the size of the load.
- Teach your children to tum the faucets off tightly after each use.
- Remember to check your sprinkler system valves periodically for
leaks and keep the heads in good shape.
- Before you lather up, install a low-flow showerhead. They’re
inexpensive, easy to install, and can save 500 gallons a week.
- Soak your pots and pans instead of letting the water run while you
scrape them clean.
- Don’t water your lawn on windy days. After all, sidewalks and
driveways do not need water.
- Water deeply but less frequently to create healthier and stronger
landscapes.
- Make sure you know where your master water shut –off valve is
located so that you can save water during any eventually.
- When watering grass on steep slopes, use a soaker hose to prevent
wastefull runoff.
- To get the most from your watering time, group your plants
according to their water needs.
- Remember to weed your lawn and garden regularly. Weeds
compete with other plants for nutrients, light, and water.
- While fertilizers promote plant growth, they also increase water
consumption. Apply the minimum amount of fertilizer needed.
- Avoid installing ornamental water features unless the water is
being recycled.
- Use a commercial car wash that recycles water.
- Don’t buy recreational water toys that require a constant flow of
water.
- Turn off the water while you brush your teeth and save 4 gallons a
minute. That’s 200 gallon a week for a family of four.
- Buy a rain gauge to track how much rain or irrigation your yard
receives.
- Encourage your community and local government to help develop
and promote a water conservation ethic.
- Teach your family how to shut off your watering systems so
anyone can turn sprinklers off when it storm is approaching.
- Set a kitchen timer when watering your lawn or garden with a
hose.
- Make sure your toilet flapper doesn’t stick open after flushing.
- Make sure there are aerators on all of your faucets.
- Choose a low water use plant for ear-round landscape color and
save up to 550 gallons each year.
- Install an instant water heater on your kitchen sink so you don’t
have to let the water run while it heats up.
- Use a grease pencil to mark the water level of your pool at the
skimmer. Check the mark 24 hours later.
- Spot spray or remove weeds as they appear.
- Use a screwdriver as a soil probe to test soil moisture. Proper lawn
watering can save thousands of gallons of water annually.
- Install a drip irrigation system around your trees and shrubs to
water more efficiently.
- Mow your lawn as infrequently as possible. Mowing puts your
lawn under additional stress, causing it to require more water.
- Don’t use the sprinklers just to cool off or for play. Running
through water from a hose or sprinkler wastes gallons of water.
- Make sure your swimming pools, fountains, and ponds are
equipped with recirculating pumps.
- Bathe your young children together.
- Direct downspouts or gutters toward shrubs or trees.
- Winterize outdoor spigots to avoid pipes from bursting or
freezing.
- Insulate hot water pipes so you don’t have to run as much water
to get hot water to the faucet.
- Drop that tissue in the trash instead of flushing it and save gallons
every time.
- Drop your tissues in the trash instead of flushing it and save
gallons every time.
- If you have an evaporative air conditioner, direct the water drain
to a flowerbed, tree, or your lawn.
- Make suggestions to your employer to save water (and dollars) at
work.
- Use a hose nozzle and turn off the water while you wash your car
to save more than 100 gallons.
- Use a hose nozzle and turn off the water while you wash your car
to save more than 100 gallons.
- Encourage your friends and neighbors to be part of a water-
conscious community.
- Install a toilet dam or bottle filled with water in your toilet to cut
down on the amount of water used for each flush.
- Install water-softening systems only when necessary. Save water
and salt by running the minimum number of regenerations.
- Wash clothes only when you have a full load and save up to 600
gallons each month.
- Prune back heavy foliage. Reducing leaf area reduces water needs.
- Report significant water losses from broken pipes and errant
sprinklers to the property owner or your water management
district.
- If your grass is brown, it’s not dead, it’s just dormant. Dormant
grass only needs to be watered every three weeks.
- Start a compost pile. Using compost when you plant adds water-
holding organic matter to the soil.
- Listen for dripping faucets and toilets that flush themselves. Fixing
a leak can save 500 gallons each month.
- Listen for dripping faucets and toilets that flush themselves. Fixing
a leak can save 500 gallons each month.
- More plants die from over-watering than from under-watering. Be
sure only to water plants when necessary.
- Adjust your watering schedule to the season. Water your summer
lawn every third day and your winter lawn every fifth day.
- Adjust your watering schedule according to the season. Water your summer
lawn every third day and your winter lawn every fifth day.
- Turn the water off while you shampoo and condition your hair
and you can save more than 50 gallons a week.
- Bathe your pets outdoors in an area in need of water.
- Choose new water-saving appliances, like washing machines that
save up to 20 gallons per load.
- Water only as rapidly as the soil can absorb the water.
- Aerate your lawn. Punch holes in your lawn about six inches apart
so water will reach the roots rather than run off the surface.
- Select the proper size pans for cooking. Large pans require more
cooking water than may be necessary.
- For lawn watering advice, contact your local conservation office.
- Turn off the water while you shave and you can save more than
100 gallons a week.
- Spread awareness and knowledge of water conservation to all your
friend and acquaintances.
There are a number of ways to save water, and they all start with you.
Rain Water Harvesting Basics
Rain water harvesting system can be as simple as an inverted umbrella that collects the rain water and directs it into a container. Alternatively, it can be a complex system that harvests every drop of rain that falls in your premises and then plus it back into your water supply system. The choice of the system depends on the size of the catchment area the amount of rainfall received, the end use of the harvested water and of course your budget. The rain water harvesting system can not only be incorporated in a new construction but can also be added to any existing structure.
Five Components of Rain Water Harvesting Systems
- Catchment Area
- Collection System
- Filtration System
- Storage / Recharge System
- Reuse System
Catchment Area
The area of your premises in which the rain water falls is the catchment area. The nature of the catchement area determines the amount and the quality of the rain water that can be harvested. The various common surfaces, have been assigned certain values of Run –off coefficients depending on the amount of rainfall that runs off their surface. The rainwater runs off the hard and smooth surfaces faster than off the soft surfaces. Hence the run off coefficient for harder surfaces is mor ethan that of the soft surfaces.
The annual rain water harvesting potential of your premises can be calculated by multiplying the respective type of area to the run-off factor and the amount of rainfall that is received annually. The ideal rain water harvesting system aims to harvest the maximum portion of this potential and achieve “zero-run-off” in your premises.
Collection System
The collection system directs the rainwater falling in your premises into the filtration system through a system of drainage pipes and channels. The collecting pipes collect the rain water falling onto the roof, running off the driveway and flowing off the other open areas and deposit it into the filtration chamber. The designing of the collection system is done in a way so as to collect the maximum of amount of run-off that is generated in the premises.
Pre-storage / Recharge Filtration
The rainwater dissolves the impurities that are present on the surface as it flows through the premises into the collection system. Therefore it is advisable to keep the catchments area free of any chemical or other harmful impurities. At times, it is also advised that the run-off of the first few minutes of the rain be allowed to flow out of the premises. This washes away most of the impurities that may be possibly present on the surfaces. However this calls for certain design modifications and vigilant users. Therefore, it is always safer to make the rainwater run-off pass through a simple filtration pit before it flows into the storage or the recharge structures. This way, most of the impurities that get dissolved in the rainwater run-off get removed before storage / recharge.
Storage / Recharge System
Depending on the amount of the water that needs to be harvested and the proposed end use of the harvested rainwaters, an appropriate storage or recharge system is designed. In areas with rainfall evenly spread over the year. Simple storage cistern can be designed on the basis of the daily water requirements. However in areas where the rainfall is restricted to a few months of the year, recharge systems are most appropriate. The design and the location of these recharge systems is site specific and needs to be evolved as per the requirements.
Re-Use System
The reuse system depends on the need of the individual owners and the amount of harvested rainwater. The harvested rainwater that has been passed through the simple pre-storage filtration system can be utilized for all uses except drinking and bathing. After adequate filtration the same water can also be made fit for human consumption. A harvested water distribution system can be worked in the premises for gardening the green areas or for use in the toilets. Again the re-use distribution system shall be site specific and the design shall be dictated by the site specification.












